Reorganization of the growth pattern of Schizosaccharomyces pombe in invasive filament formation
- PMID: 20870879
- PMCID: PMC2976291
- DOI: 10.1128/EC.00084-10
Reorganization of the growth pattern of Schizosaccharomyces pombe in invasive filament formation
Abstract
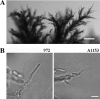
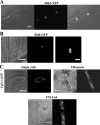
The organization and control of polarized growth through the cell cycle of Schizosaccharomyces pombe, a single-celled eukaryote, have been studied extensively. We have investigated the changes in these processes when S. pombe differentiates to form multicellular invasive mycelia and have found striking alterations to the behavior of some of the key regulatory proteins. Cells at the tips of invading filaments are considerably more elongated than cells growing singly and grow at one pole only. The filament tip follows a strict direction of growth through multiple cell cycles. A group of proteins involved in the growth process and actin regulation, comprising Spo20, Bgs4, activated Cdc42, and Crn1, are all concentrated at the growing tip, unlike their distribution at both ends of single cells. In contrast, several proteins implicated in microtubule-dependent organization of growth, including Tea1, Tea4, Mod5, and Pom1, all show the opposite effect and are relatively depleted at the growing end and enriched at the nongrowing end, although Tea1 appears to continue to be delivered to both ends. A third group acting at different stages of the cell cycle, including Bud6, Rga4, and Mid1, localize similarly in filaments and single cells, while Nif1 shows a reciprocal localization to Pom1.
Figures





Similar articles
-
Local and global Cdc42 guanine nucleotide exchange factors for fission yeast cell polarity are coordinated by microtubules and the Tea1-Tea4-Pom1 axis.J Cell Sci. 2018 Jul 19;131(14):jcs216580. doi: 10.1242/jcs.216580. J Cell Sci. 2018. PMID: 29930085 Free PMC article.
-
Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast.Curr Biol. 2008 Mar 11;18(5):322-30. doi: 10.1016/j.cub.2008.02.005. Curr Biol. 2008. PMID: 18328707 Free PMC article.
-
A catalytic role for Mod5 in the formation of the Tea1 cell polarity landmark.Curr Biol. 2010 Oct 12;20(19):1752-7. doi: 10.1016/j.cub.2010.08.035. Epub 2010 Sep 16. Curr Biol. 2010. PMID: 20850323 Free PMC article.
-
Wsh3/Tea4 is a novel cell-end factor essential for bipolar distribution of Tea1 and protects cell polarity under environmental stress in S. pombe.Curr Biol. 2005 Jun 7;15(11):1006-15. doi: 10.1016/j.cub.2005.04.061. Curr Biol. 2005. PMID: 15936270
-
Microtubule-dependent cell morphogenesis in the fission yeast.Trends Cell Biol. 2009 Sep;19(9):447-54. doi: 10.1016/j.tcb.2009.06.003. Epub 2009 Aug 25. Trends Cell Biol. 2009. PMID: 19713114 Review.
Cited by
-
Understanding the molecular mechanisms of human diseases: the benefits of fission yeasts.Microb Cell. 2024 Aug 2;11:288-311. doi: 10.15698/mic2024.08.833. eCollection 2024. Microb Cell. 2024. PMID: 39104724 Free PMC article.
-
Filamentous invasive growth of mutants of the genes encoding ammonia-metabolizing enzymes in the fission yeast Schizosaccharomyces pombe.PLoS One. 2017 Oct 5;12(10):e0186028. doi: 10.1371/journal.pone.0186028. eCollection 2017. PLoS One. 2017. PMID: 28982178 Free PMC article.
-
A Genetic Screen for Fission Yeast Gene Deletion Mutants Exhibiting Hypersensitivity to Latrunculin A.G3 (Bethesda). 2016 Oct 13;6(10):3399-3408. doi: 10.1534/g3.116.032664. G3 (Bethesda). 2016. PMID: 27466272 Free PMC article.
-
Phosphoregulation of the cytokinetic protein Fic1 contributes to fission yeast growth polarity establishment.J Cell Sci. 2020 Sep 17;133(18):jcs244392. doi: 10.1242/jcs.244392. J Cell Sci. 2020. PMID: 32878942 Free PMC article.
-
Fungal evolution: major ecological adaptations and evolutionary transitions.Biol Rev Camb Philos Soc. 2019 Aug;94(4):1443-1476. doi: 10.1111/brv.12510. Epub 2019 Apr 25. Biol Rev Camb Philos Soc. 2019. PMID: 31021528 Free PMC article. Review.
References
-
- Cortes J. C. G., Konomi M., Martins I. M., Munoz J., Moreno M. B., Osumi M., Duran A., Ribas J. C. 2007. The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 65:201–217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

