α-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa
- PMID: 20870880
- PMCID: PMC2976294
- DOI: 10.1128/EC.00134-10
α-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa
Abstract
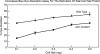
The enzyme α-1,6-mannosyltransferase (OCH-1) is required for the synthesis of galactomannans attached to the N-linked oligosaccharides of Neurospora crassa cell wall proteins. The Neurospora crassa och-1 mutant has a tight colonial phenotype and a defective cell wall. A carbohydrate analysis of the och-1 mutant cell wall revealed a 10-fold reduction in the levels of mannose and galactose and a total lack of 1,6-linked mannose residues. Analysis of the integral cell wall protein from wild-type and och-1 mutant cells showed that the mutant cell wall had reduced protein content. The och-1 mutant was found to secrete 18-fold more protein than wild-type cells. Proteomic analysis of the proteins released by the mutant into the growth medium identified seven of the major cell wall proteins. Western blot analysis of ACW-1 and GEL-1 (two glycosylphosphatidylinositol [GPI]-anchored proteins that are covalently integrated into the wild-type cell wall) showed that high levels of these proteins were being released into the medium by the och-1 mutant. High levels of ACW-1 and GEL-1 were also released from the och-1 mutant cell wall by subjecting the wall to boiling in a 1% SDS solution, indicating that these proteins are not being covalently integrated into the mutant cell wall. From these results, we conclude that N-linked mannosylation of cell wall proteins by OCH-1 is required for their efficient covalent incorporation into the cell wall.
Figures




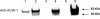

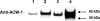
Similar articles
-
Mutational analysis of the glycosylphosphatidylinositol (GPI) anchor pathway demonstrates that GPI-anchored proteins are required for cell wall biogenesis and normal hyphal growth in Neurospora crassa.Eukaryot Cell. 2006 Mar;5(3):587-600. doi: 10.1128/EC.5.3.587-600.2006. Eukaryot Cell. 2006. PMID: 16524913 Free PMC article.
-
Mannosyltransferase is required for cell wall biosynthesis, morphology and control of asexual development in Neurospora crassa.Mycologia. 2005 Jul-Aug;97(4):872-9. doi: 10.3852/mycologia.97.4.872. Mycologia. 2005. PMID: 16457356
-
The Predicted Mannosyltransferase GT69-2 Antagonizes RFW-1 To Regulate Cell Fusion in Neurospora crassa.mBio. 2021 Mar 16;12(2):e00307-21. doi: 10.1128/mBio.00307-21. mBio. 2021. PMID: 33727349 Free PMC article.
-
White collar proteins: PASsing the light signal in Neurospora crassa.Trends Microbiol. 1997 Nov;5(11):458-62. doi: 10.1016/S0966-842X(97)01144-X. Trends Microbiol. 1997. PMID: 9402704 Review.
-
The Genetics and Biochemistry of Cell Wall Structure and Synthesis in Neurospora crassa, a Model Filamentous Fungus.Front Microbiol. 2019 Oct 10;10:2294. doi: 10.3389/fmicb.2019.02294. eCollection 2019. Front Microbiol. 2019. PMID: 31649638 Free PMC article. Review.
Cited by
-
A proteomic and genetic analysis of the Neurospora crassa conidia cell wall proteins identifies two glycosyl hydrolases involved in cell wall remodeling.Fungal Genet Biol. 2016 Sep;94:47-53. doi: 10.1016/j.fgb.2016.07.003. Epub 2016 Jul 2. Fungal Genet Biol. 2016. PMID: 27381444 Free PMC article.
-
Trichophyton rubrum LysM proteins bind to fungal cell wall chitin and to the N-linked oligosaccharides present on human skin glycoproteins.PLoS One. 2019 Apr 4;14(4):e0215034. doi: 10.1371/journal.pone.0215034. eCollection 2019. PLoS One. 2019. PMID: 30947244 Free PMC article.
-
Characterization of PbPga1, an antigenic GPI-protein in the pathogenic fungus Paracoccidioides brasiliensis.PLoS One. 2012;7(9):e44792. doi: 10.1371/journal.pone.0044792. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024763 Free PMC article.
-
The Neurospora crassa dfg5 and dcw1 genes encode α-1,6-mannanases that function in the incorporation of glycoproteins into the cell wall.PLoS One. 2012;7(6):e38872. doi: 10.1371/journal.pone.0038872. Epub 2012 Jun 11. PLoS One. 2012. PMID: 22701726 Free PMC article.
-
Functional production of human antibody by the filamentous fungus Aspergillus oryzae.Fungal Biol Biotechnol. 2020 May 28;7:7. doi: 10.1186/s40694-020-00098-w. eCollection 2020. Fungal Biol Biotechnol. 2020. PMID: 32514366 Free PMC article.
References
-
- Ballou C. E. 1990. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 185:440–479 - PubMed
-
- Barnay-Verdier S., Boisrame A., Beckerick J.-M. 2004. Identification and characterization of two α-1,6-mannosyltransferases, Anl1p and Och1p, in the yeast Yarrowia lipolytica. Microbiology 150:2185–2195 - PubMed
-
- Bates S., Hughes H. B., Munro C. A., Thomas W. P. H., MacCallum D. M., Bertram G., et al. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90–98 - PubMed
-
- Bouffard F. A., Zambias R. A., Dropinski J. F., Balkovec J. M., Hammond M. L., Abrazzo G. K., et al. 1994. Synthesis and antifungal activity of novel cationic pneumocandin B(o) derivatives. J. Med. Chem. 37:222–225 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

