Anti-inflammatory effects of thiazolidinediones in human airway smooth muscle cells
- PMID: 20870897
- PMCID: PMC3145064
- DOI: 10.1165/rcmb.2009-0445OC
Anti-inflammatory effects of thiazolidinediones in human airway smooth muscle cells
Abstract
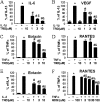
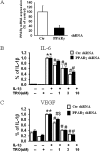
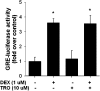
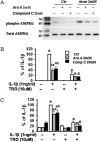
Airway smooth muscle (ASM) cells have been reported to contribute to the inflammation of asthma. Because the thiazolidinediones (TZDs) exert anti-inflammatory effects, we examined the effects of troglitazone and rosiglitazone on the release of inflammatory moieties from cultured human ASM cells. Troglitazone dose-dependently reduced the IL-1β-induced release of IL-6 and vascular endothelial growth factor, the TNF-α-induced release of eotaxin and regulated on activation, normal T expressed and secreted (RANTES), and the IL-4-induced release of eotaxin. Rosiglitazone also inhibited the TNF-α-stimulated release of RANTES. Although TZDs are known to activate peroxisome proliferator-activated receptor-γ (PPARγ), these anti-inflammatory effects were not affected by a specific PPARγ inhibitor (GW 9662) or by the knockdown of PPARγ using short hairpin RNA. Troglitazone and rosiglitazone each caused the activation of adenosine monophosphate-activated protein kinase (AMPK), as detected by Western blotting using a phospho-AMPK antibody. The anti-inflammatory effects of TZDs were largely mimicked by the AMPK activators, 5-amino-4-imidazolecarboxamide ribose (AICAR) and metformin. However, the AMPK inhibitors, Ara A and Compound C, were not effective in preventing the anti-inflammatory effects of troglitazone or rosiglitzone, suggesting that the effects of these TZDs are likely not mediated through the activation of AMPK. These data indicate that TZDs inhibit the release of a variety of inflammatory mediators from human ASM cells, suggesting that they may be useful in the treatment of asthma, and the data also indicate that the effects of TZDs are not mediated by PPARγ or AMPK.
Figures



References
-
- Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy 2008;38:1847–1857. - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990;323:1033–1039. - PubMed
-
- Ghaffar O, Hamid Q, Renzi PM, Allakhverdi Z, Molet S, Hogg JC, Shore SA, Luster AD, Lamkhioued B. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am J Respir Crit Care Med 1999;159:1933–1942. - PubMed
-
- Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, Jones MR, Mizgerd JP, Whitehead T, Imrich A, et al. Oncostatin M causes eotaxin-1 release from airway smooth muscle: synergy with IL-4 and IL-13. J Allergy Clin Immunol 2005;115:514–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

