Molecular intercommunication between the complement and coagulation systems
- PMID: 20870944
- PMCID: PMC3123139
- DOI: 10.4049/jimmunol.0903678
Molecular intercommunication between the complement and coagulation systems
Abstract
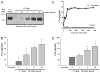
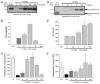
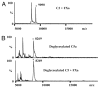
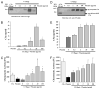
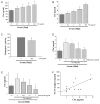
The complement system as well as the coagulation system has fundamental clinical implications in the context of life-threatening tissue injury and inflammation. Associations between both cascades have been proposed, but the precise molecular mechanisms remain unknown. The current study reports multiple links for various factors of the coagulation and fibrinolysis cascades with the central complement components C3 and C5 in vitro and ex vivo. Thrombin, human coagulation factors (F) XIa, Xa, and IXa, and plasmin were all found to effectively cleave C3 and C5. Mass spectrometric analyses identified the cleavage products as C3a and C5a, displaying identical molecular weights as the native anaphylatoxins C3a and C5a. Cleavage products also exhibited robust chemoattraction of human mast cells and neutrophils, respectively. Enzymatic activity for C3 cleavage by the investigated clotting and fibrinolysis factors is defined in the following order: FXa > plasmin > thrombin > FIXa > FXIa > control. Furthermore, FXa-induced cleavage of C3 was significantly suppressed in the presence of the selective FXa inhibitors fondaparinux and enoxaparin in a concentration-dependent manner. Addition of FXa to human serum or plasma activated complement ex vivo, represented by the generation of C3a, C5a, and the terminal complement complex, and decreased complement hemolytic serum activity that defines exact serum concentration that results in complement-mediated lysis of 50% of sensitized sheep erythrocytes. Furthermore, in plasma from patients with multiple injuries (n = 12), a very early appearance and correlation of coagulation (thrombin-antithrombin complexes) and the complement activation product C5a was found. The present data suggest that coagulation/fibrinolysis proteases may act as natural C3 and C5 convertases, generating biologically active anaphylatoxins, linking both cascades via multiple direct interactions in terms of a complex serine protease system.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
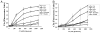

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

