The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation
- PMID: 20870962
- PMCID: PMC2965533
- DOI: 10.1105/tpc.110.074351
The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation
Abstract
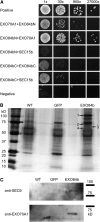
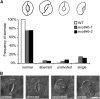
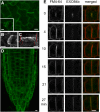
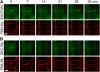
Cell reproduction is a complex process involving whole cell structures and machineries in space and time, resulting in regulated distribution of endomembranes, organelles, and genomes between daughter cells. Secretory pathways supported by the activity of the Golgi apparatus play a crucial role in cytokinesis in plants. From the onset of phragmoplast initiation to the maturation of the cell plate, delivery of secretory vesicles is necessary to sustain successful daughter cell separation. Tethering of secretory vesicles at the plasma membrane is mediated by the evolutionarily conserved octameric exocyst complex. Using proteomic and cytologic approaches, we show that EXO84b is a subunit of the plant exocyst. Arabidopsis thaliana mutants for EXO84b are severely dwarfed and have compromised leaf epidermal cell and guard cell division. During cytokinesis, green fluorescent protein-tagged exocyst subunits SEC6, SEC8, SEC15b, EXO70A1, and EXO84b exhibit distinctive localization maxima at cell plate initiation and cell plate maturation, stages with a high demand for vesicle fusion. Finally, we present data indicating a defect in cell plate assembly in the exo70A1 mutant. We conclude that the exocyst complex is involved in secretory processes during cytokinesis in Arabidopsis cells, notably in cell plate initiation, cell plate maturation, and formation of new primary cell wall.
Figures





References
-
- Abramoff M.D., Magelhaes P.J., Ram S.J. (2004). Image Processing with ImageJ. Biophotonics Int. 11: 36–42
-
- Buschmann H., Chan J., Sanchez-Pulido L., Andrade-Navarro M.A., Doonan J.H., Lloyd C.W. (2006). Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Curr. Biol. 16: 1938–1943 - PubMed
-
- Chiu W., Niwa Y., Zeng W., Hirano T., Kobayashi H., Sheen J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6: 325–330 - PubMed
-
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

