A simple fluorogenic method for determination of acid ceramidase activity and diagnosis of Farber disease
- PMID: 20871013
- PMCID: PMC2975727
- DOI: 10.1194/jlr.D010033
A simple fluorogenic method for determination of acid ceramidase activity and diagnosis of Farber disease
Abstract
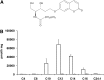
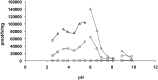
Acid ceramidase (aCDase) is one of several enzymes responsible for ceramide degradation within mammalian cells. As such, aCDase regulates the intracellular levels of the bioactive lipid ceramide. An inherited deficiency of aCDase activity results in Farber disease (FD), also called lipogranulomatosis, which is characterized by ceramide accumulation in the tissues of patients. Diagnosis of FD is confirmed by demonstration of a deficient aCDase activity and the subsequent storage of ceramide. Existing methods include extremely complex assays, many of them using radiolabeled compounds. Therefore, the aCDase assay and the in vitro enzymatic diagnosis of FD are still performed in only a very limited number of specialized laboratories. Here, the new fluorogenic substrate Rbm14-12 was synthesized and characterized as a new tool to determine aCDase activity. The resulting optimized assay was performed in 96-well plates, and different fibroblast and lymphoid cell lines derived from FD patients and controls were tested to measure aCDase activity. As a result, the activity in cells of FD patients was found to be very low or even null. This new fluorogenic method offers a very easy and rapid way for specific and accurate determination of aCDase activity and, consequently, for diagnosis of FD.
Figures

References
-
- Moser H. W., Moser A. B., Chen W. W., Schram A. W. 1989. Ceramidase deficiency: Farber lipogranulomatosis. The metabolic basis of inherited disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., McGraw-Hill, New York: 1645–1654.
-
- Levade T., Sandhoff K., Schulze H., Medin J. A. 2009. Acid ceramidase deficiency: Farber lipogranulomatosis. In Scriver's OMMBID (Online Metabolic and Molecular Bases of Inherited Disease), Valle D., et al., New York: McGraw-Hill; http://www.ommbid.com
-
- Nilsson A. 1969. The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim. Biophys. Acta. 176: 339–347. - PubMed
-
- Sugita M., Willians M., Dulaney J. T., Moser H. W. 1975. Ceramidase and ceramide synthesis in human kidney and cerebellum. Description of a new alkaline ceramidase. Biochim. Biophys. Acta. 398: 125–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

