Certolizumab pegol in the treatment of rheumatoid arthritis: a comprehensive review of its clinical efficacy and safety
- PMID: 20871129
- PMCID: PMC3021948
- DOI: 10.1093/rheumatology/keq285
Certolizumab pegol in the treatment of rheumatoid arthritis: a comprehensive review of its clinical efficacy and safety
Abstract
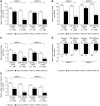
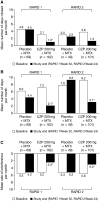
Biological agents, including TNF inhibitors, have revolutionized the treatment of RA in recent years. Certolizumab pegol (CZP) is a novel pegylated anti-TNF approved for the treatment of adult patients with moderately to severely active RA. This article provides an overview of three published clinical trials of CZP in RA in patients with active disease who have shown an inadequate response to DMARDs, including MTX: RA prevention of structural damage (RAPID) 1 and 2, which evaluated the efficacy and safety of CZP added to MTX when dosed every 2 weeks, and efficacy and safety of CZP - 4 weekly dosage in rheumatoid arthritis (FAST4WARD), which evaluated CZP monotherapy when dosed every 4 weeks. In the trials, CZP plus MTX or as monotherapy significantly improved the signs and symptoms of RA and RA disease activity, and CZP plus MTX significantly inhibited the progression of radiographic joint damage as early as Week 16 of the treatment. In addition, CZP treatment significantly improved patient-reported outcome measures, providing significant reductions in pain and fatigue and improvements in physical function as early as Week 1 of treatment; improvements in health-related quality of life were evident at the first assessment at Week 12. CZP treatment improved productivity at work, significantly reducing the number of days of missed work as well as the number of days with reduced productivity, and also increased productivity within the home and improved participation in family, social and leisure activities. CZP was generally well tolerated when used either as monotherapy or added to MTX; most adverse events were mild or moderate. Taken together, the results of these trials suggest that CZP is an effective new option for the treatment of RA.
Figures

Similar articles
-
Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J-RAPID randomized, placebo-controlled trial.Mod Rheumatol. 2014 Sep;24(5):715-24. doi: 10.3109/14397595.2013.864224. Epub 2013 Dec 9. Mod Rheumatol. 2014. PMID: 24313916 Clinical Trial.
-
Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo-controlled trial.Mod Rheumatol. 2014 Jul;24(4):552-60. doi: 10.3109/14397595.2013.843764. Epub 2013 Nov 1. Mod Rheumatol. 2014. PMID: 24981319 Clinical Trial.
-
Effect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritis.Arthritis Rheum. 2009 Nov 15;61(11):1592-600. doi: 10.1002/art.24828. Arthritis Rheum. 2009. PMID: 19877104 Clinical Trial.
-
Efficacy and safety of certolizumab pegol in rheumatoid arthritis: meeting rheumatologists' requirements in routine clinical practice.BioDrugs. 2014 Apr;28 Suppl 1:S25-37. doi: 10.1007/s40259-013-0065-y. BioDrugs. 2014. PMID: 24687236 Review.
-
Certolizumab pegol (CIMZIA®) for the treatment of rheumatoid arthritis.Health Technol Assess. 2010 Oct;14(Suppl. 2):1-10. doi: 10.3310/hta14suppl2/01. Health Technol Assess. 2010. PMID: 21047485 Review.
Cited by
-
The efficacy of certolizumab pegol in rheumatoid arthritis assessed by gray scale and power Doppler ultrasonography: case reports.Drugs Context. 2019 Feb 13;8:212554. doi: 10.7573/dic.212554. eCollection 2019. Drugs Context. 2019. PMID: 30828352 Free PMC article. No abstract available.
-
Regulation of tumour necrosis factor signalling: live or let die.Nat Rev Immunol. 2015 Jun;15(6):362-74. doi: 10.1038/nri3834. Nat Rev Immunol. 2015. PMID: 26008591 Review.
-
Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development.Cells. 2018 Oct 9;7(10):161. doi: 10.3390/cells7100161. Cells. 2018. PMID: 30304822 Free PMC article. Review.
-
Potential applications and human biosafety of nanomaterials used in nanomedicine.J Appl Toxicol. 2018 Jan;38(1):3-24. doi: 10.1002/jat.3476. Epub 2017 Jun 6. J Appl Toxicol. 2018. PMID: 28589558 Free PMC article. Review.
-
Paradoxical inflammation induced by anti-TNF agents in patients with IBD.Nat Rev Gastroenterol Hepatol. 2012 Sep;9(9):496-503. doi: 10.1038/nrgastro.2012.125. Epub 2012 Jul 3. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22751454 Review.
References
-
- Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398–408. - PubMed
-
- Palframan R, Airey M, Moore A, Vugler A, Nesbitt A. Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J Immunol Methods. 2009;348:36–41. - PubMed
-
- Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–32. - PubMed
-
- Keystone E, van der Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

