Phase II study of the histone deacetylase inhibitor Romidepsin in relapsed small cell lung cancer (Cancer and Leukemia Group B 30304)
- PMID: 20871263
- PMCID: PMC3782083
- DOI: 10.1097/JTO.0b013e3181ec1713
Phase II study of the histone deacetylase inhibitor Romidepsin in relapsed small cell lung cancer (Cancer and Leukemia Group B 30304)
Abstract
Introduction: Treatment of small cell lung cancer (SCLC) is initially gratifying with most patients responding to platinum-based chemotherapy. Treatment of relapsed disease gives much lower response rates of short duration. We undertook this study of the protein deacetylase inhibitor Romidepsin in chemosensitive recurrent SCLC based on preclinical data that suggested this to be an active target.
Methods: Patients had recurrent chemosensitive SCLC (relapse ≥90 days since completion of platinum-based chemotherapy). Treatment was administered as weekly infusions of Romidepsin at 13 mg/m(2) for 3 of 4 weeks. We designed a two-stage phase II study targeting a response rate of 30% (<10% response would be uninteresting and ≥30% worthy of further study).
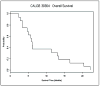
Results: Sixteen patients (10 male, 6 female) were accrued to the first stage of this study. Most (11 patients, 69%) presented with extensive-stage SCLC, and all had received prior chemotherapy, with 11 having received prior radiation. Eastern Cooperative Oncology Group performance status was excellent with 0 in 6 patients (38%) and 1 in 10 patients. No objective responses were seen, and stable disease was the best response seen in 3 patients (19%). Toxicity was modest with 3 patients suffering grade 3 toxicity (lymphopenia, insomnia, nausea, vomiting, and hyponatremia) and one patient with grade 4 thrombocytopenia. Median progression-free survival was 1.8 months, and median overall survival was 6 months.
Conclusion: Romidepsin given on a weekly schedule in patients with chemosensitive, recurrent SCLC was inactive and will not be pursued further in this setting.
Figures

Similar articles
-
A phase II study of dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602).J Thorac Oncol. 2010 Mar;5(3):380-4. doi: 10.1097/JTO.0b013e3181cee36e. J Thorac Oncol. 2010. PMID: 20087228 Free PMC article. Clinical Trial.
-
Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer.J Thorac Oncol. 2010 Oct;5(10):1637-43. doi: 10.1097/JTO.0b013e3181e8f4dc. J Thorac Oncol. 2010. PMID: 20808253 Clinical Trial.
-
Phase II study of bendamustine in relapsed chemotherapy sensitive or resistant small-cell lung cancer.J Thorac Oncol. 2014 Apr;9(4):559-62. doi: 10.1097/JTO.0000000000000079. J Thorac Oncol. 2014. PMID: 24736081 Free PMC article. Clinical Trial.
-
Phase II trial of romidepsin (NSC-630176) in previously treated colorectal cancer patients with advanced disease: a Southwest Oncology Group study (S0336).Invest New Drugs. 2009 Oct;27(5):469-75. doi: 10.1007/s10637-008-9190-8. Epub 2008 Oct 22. Invest New Drugs. 2009. PMID: 18941712 Free PMC article. Clinical Trial.
-
Romidepsin for the treatment of T-cell lymphomas.Am J Health Syst Pharm. 2013 Jul 1;70(13):1115-22. doi: 10.2146/ajhp120163. Am J Health Syst Pharm. 2013. PMID: 23784158 Review.
Cited by
-
Epigenetics in cancer stem cells.Mol Cancer. 2017 Feb 1;16(1):29. doi: 10.1186/s12943-017-0596-9. Mol Cancer. 2017. PMID: 28148257 Free PMC article. Review.
-
Beyond regulations at DNA levels: A review of epigenetic therapeutics targeting cancer stem cells.Cell Prolif. 2021 Feb;54(2):e12963. doi: 10.1111/cpr.12963. Epub 2020 Dec 13. Cell Prolif. 2021. PMID: 33314500 Free PMC article. Review.
-
Epigenetic Therapy for Solid Tumors: Highlighting the Impact of Tumor Hypoxia.Genes (Basel). 2015 Sep 25;6(4):935-56. doi: 10.3390/genes6040935. Genes (Basel). 2015. PMID: 26426056 Free PMC article. Review.
-
Strategies to Target Chemoradiotherapy Resistance in Small Cell Lung Cancer.Cancers (Basel). 2024 Oct 10;16(20):3438. doi: 10.3390/cancers16203438. Cancers (Basel). 2024. PMID: 39456533 Free PMC article. Review.
-
Phase I and Pharmacokinetic Study of Romidepsin in Patients with Cancer and Hepatic Dysfunction: A National Cancer Institute Organ Dysfunction Working Group Study.Clin Cancer Res. 2020 Oct 15;26(20):5329-5337. doi: 10.1158/1078-0432.CCR-20-1412. Epub 2020 Aug 14. Clin Cancer Res. 2020. PMID: 32816943 Free PMC article. Clinical Trial.
References
-
- Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. - PubMed
-
- Murray N, Turrisi AT., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1(3):270–8. - PubMed
-
- Cheng S, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2007;2(4):348–54. - PubMed
-
- Socinski MA, Bogart JA. Limited-stage small-cell lung cancer: the current status of combined-modality therapy. J Clin Oncol. 2007;25(26):4137–45. - PubMed
-
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83(3):355–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA077658/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- U10 CA114558/CA/NCI NIH HHS/United States
- U10 CA045418/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA045389/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA077597/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

