Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny
- PMID: 20871424
- PMCID: PMC3774031
- DOI: 10.1227/NEU.0b013e3181f556ab
Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny
Abstract
Background: Identifying the origin of gliomas carries important implications for advancing the treatment of these recalcitrant tumors. Recent research promotes the hypothesis of a subventricular zone (SVZ) origin for the stemlike gliomagenic cells identified within human glioma specimens. However, conflicting evidence suggests that SVZ-like cells are not uniquely gliomagenic but this capacity may be shared by cycling progenitors distributed throughout the subcortical white matter (SCWM).
Objective: To review radiological evidence in glioblastoma multiforme (GBM) patients to provide insight into the question of glioma ontogeny.
Methods: We explored whether GBMs at first diagnosis demonstrated a pattern of anatomic distribution consistent with origin at the SVZ through retrospective analysis of preoperative contrast-enhanced T1-weighted magnetic resonance images in 63 patients. We then examined the relationship of tumor volume, point of origin, and proximity to the ventricles using a computer model of glioma growth.
Results: Fewer than half of the GBMs analyzed had contrast-enhancing portions that contacted the ventricle on preoperative imaging. A strong correlation was found between tumor volume and the distance between the contrast-enhancing edge of the tumor and the ventricle, demonstrating that tumors abutting the ventricle are significantly larger than those that do not. The lesions simulated by the computer model validated our assumption that tumors that are radiographically distant from the ventricles are unlikely to have originated in the SVZ and supported our hypothesis that as they grow, the edges of all tumors will near the ventricles, regardless of their point of origin.
Conclusion: This work offers further support for the hypothesis that the origins of GBMs are at sites distributed throughout the white matter and are not limited to the region of the SVZ.
Conflict of interest statement
This work was made possible in part by a Doris Duke Clinical Research Fellowship (Dr Bohman), National Institutes of Health grant RO1 CA 89395 (Dr Bruce), a Mary Gates Research Scholarship (J.L. Moore), and a 21st Century Scientist Award from the McDonnell Foundation (Dr Swanson and R. Rockne). The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Figures
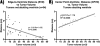




References
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. - PubMed
-
- Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64(14):4783–4789. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. - PubMed
-
- Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5(11):899–904. - PubMed
-
- Oliver TG, Wechsler-Reya RJ. Getting at the root and stem of brain tumors. Neuron. 2004;42(6):885–888. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

