Replication of individual DNA molecules under electronic control using a protein nanopore
- PMID: 20871614
- PMCID: PMC3711841
- DOI: 10.1038/nnano.2010.177
Replication of individual DNA molecules under electronic control using a protein nanopore
Abstract
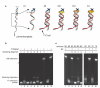
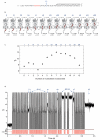
Nanopores can be used to analyse DNA by monitoring ion currents as individual strands are captured and driven through the pore in single file by an applied voltage. Here, we show that serial replication of individual DNA templates can be achieved by DNA polymerases held at the α-haemolysin nanopore orifice. Replication is blocked in the bulk phase, and is initiated only after the DNA is captured by the nanopore. We used this method, in concert with active voltage control, to observe DNA replication catalysed by bacteriophage T7 DNA polymerase (T7DNAP) and by the Klenow fragment of DNA polymerase I (KF). T7DNAP advanced on a DNA template against an 80-mV load applied across the nanopore, and single nucleotide additions were measured on the millisecond timescale for hundreds of individual DNA molecules in series. Replication by KF was not observed when this enzyme was held on top of the nanopore orifice at an applied potential of 80 mV. Sequential nucleotide additions by KF were observed upon applying controlled voltage reversals.
Figures
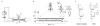



References
-
- Gyarfas B, et al. Mapping the Position of DNA Polymerase-Bound DNA Templates in a Nanopore at 5 A Resolution. ACS Nano. 2009;3:1457–1466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

