Covalent Polymers Containing Discrete Heterocyclic Anion Receptors
- PMID: 20871791
- PMCID: PMC2943640
- DOI: 10.1007/7081_2010_39
Covalent Polymers Containing Discrete Heterocyclic Anion Receptors
Abstract
This chapter covers recent advances in the development of polymeric materials containing discrete heterocyclic anion receptors, and focuses on advances in anion binding and chemosensor chemistry. The development of polymers specific for anionic species is a relatively new and flourishing area of materials chemistry. The incorporation of heterocyclic receptors capable of complexing anions through non-covalent interactions (e.g., hydrogen bonding and electrostatic interactions) provides a route to not only sensitive but also selective polymer materials. Furthermore, these systems have been utilized in the development of polymers capable of extracting anionic species from aqueous environments. These latter materials may lead to advances in water purification and treatment of diseases resulting from surplus ions.
Figures

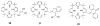

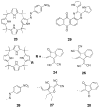
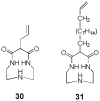
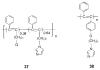
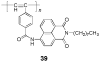
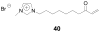


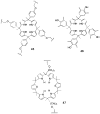





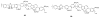




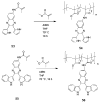
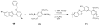
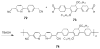
References
-
- Staudinger H. Ber Dtsch Chem Ges. 1920;53B:1073–1085.
-
- Staudinger H. Ber Dtsch Chem Ges. 1924;57B:1203–1208.
-
- Carothers WH, Coffman DD. J Am Chem Soc. 1932;54:4071–4076.
-
- Carothers WH. J Ind Eng Chem. 1934;26:30–33.
-
- Urry DW, Parker TM, Reid MC, Gowda DC. J Bioact Compat Polym. 1991;3:263–282.
Grants and funding
LinkOut - more resources
Full Text Sources
