An Overview of Stereoselective Synthesis of α-Aminophosphonic Acids and Derivatives
- PMID: 20871799
- PMCID: PMC2943650
- DOI: 10.1016/j.tet.2008.09.083
An Overview of Stereoselective Synthesis of α-Aminophosphonic Acids and Derivatives
Abstract
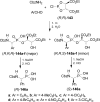
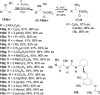
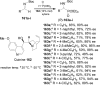
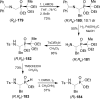
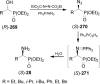
An overview of all methodologies published during the last few years focused to the stereoselective (diastereoselective or enantioselective) synthesis of α-aminophosphonic acids and derivatives is reported. The procedures have been classified according a retrosynthetic strategy and taking into account the formation of each one of the bonds connected to the chiral centre.
Figures


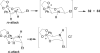


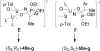
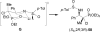


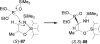

























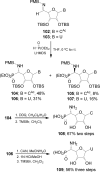

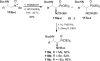
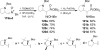
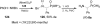

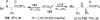




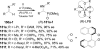


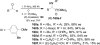












































Similar articles
-
Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives.Molecules. 2021 May 27;26(11):3202. doi: 10.3390/molecules26113202. Molecules. 2021. PMID: 34071844 Free PMC article. Review.
-
Diastereoselective hydrophosphonylation of imines using (R,R)-TADDOL phosphite. Asymmetric synthesis of alpha-aminophosphonic acid derivatives.Org Biomol Chem. 2010 Oct 7;8(19):4255-8. doi: 10.1039/c003004j. Epub 2010 Jul 29. Org Biomol Chem. 2010. PMID: 20672154
-
The Synthesis of α-Aminophosphonates via Enantioselective Organocatalytic Reaction of 1-(N-Acylamino)alkylphosphonium Salts with Dimethyl Phosphite.Molecules. 2020 Jan 18;25(2):405. doi: 10.3390/molecules25020405. Molecules. 2020. PMID: 31963713 Free PMC article.
-
Calix[4]arene alpha-aminophosphonic acids: asymmetric synthesis and enantioselective inhibition of an alkaline phosphatase.Org Lett. 2006 Feb 16;8(4):549-52. doi: 10.1021/ol052469a. Org Lett. 2006. PMID: 16468708
-
Recent synthesis of aminophosphonic acids as potential biological importance.Amino Acids. 2010 Jan;38(1):23-30. doi: 10.1007/s00726-009-0254-7. Epub 2009 Feb 20. Amino Acids. 2010. PMID: 19229586 Review.
Cited by
-
The synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik-Fields reaction.RSC Adv. 2018 Sep 7;8(55):31496-31501. doi: 10.1039/c8ra05983g. eCollection 2018 Sep 5. RSC Adv. 2018. PMID: 35548197 Free PMC article.
-
Pyrrolidine and oxazolidine ring transformations in proline and serine derivatives of α-hydroxyphosphonates induced by deoxyfluorinating reagents.RSC Adv. 2018 Jul 6;8(43):24444-24457. doi: 10.1039/c8ra05186k. eCollection 2018 Jul 2. RSC Adv. 2018. PMID: 35539185 Free PMC article.
-
Aqua-soluble DDQ reduces the levels of Drp1 and Aβ and inhibits abnormal interactions between Aβ and Drp1 and protects Alzheimer's disease neurons from Aβ- and Drp1-induced mitochondrial and synaptic toxicities.Hum Mol Genet. 2017 Sep 1;26(17):3375-3395. doi: 10.1093/hmg/ddx226. Hum Mol Genet. 2017. PMID: 28854701 Free PMC article.
-
Regioselective α-Phosphonylation of Unprotected Alicyclic Amines.Org Lett. 2024 Jul 19;26(28):5972-5977. doi: 10.1021/acs.orglett.4c02037. Epub 2024 Jul 5. Org Lett. 2024. PMID: 38968591 Free PMC article.
-
Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes.Angew Chem Int Ed Engl. 2010;49(1):153-6. doi: 10.1002/anie.200904779. Angew Chem Int Ed Engl. 2010. PMID: 19950156 Free PMC article.
References
-
- Vassiliou S, Xeilari M, Yiotakis A, Germbecka J, Pawełczak M, Kafarski P, Mucha A. Bioorg. Med. Chem. Lett. 2007;15:3187–3200. - PubMed
- El Kaïm L, Grimaud L, Hadrot S. Tetrahedron Lett. 2006;47:3945–3947.
- Cristau H-J, Coulombeau A, Genevois-Borella A, Pirat J-L. Tetrahedron Lett. 2001;42:4491–4494.
- Kukhar VP, Hudson HR, editors. Aminophosphonic and Aminophosphinic Acids. John Wiley & Sons; 2000.
- De Lombaert S, Blanchard L, Stamfort LB, Tan J, Wallace EM, Satoh Y, Fitt J, Hoyer D, Simonsbergen D, Moliterni J, Marcopoulos N, Savage P, Chou M, Trapani AJ, Jeng AY. J. Med. Chem. 2000;43:488–504. - PubMed
- De Lombaert S, Stamfort LB, Blanchard L, Tan J, Hoyer D, Diefenbacher CG, Wei D, Wallace EM, Moskal MA, Savage P, Jeng AY. Bioorg. Med. Chem. Lett. 1997;7:1059–1064.
- Lloyd J, Schmidt JB, Hunt JT, Barrish JC, Little DK, Tymiak AA. Bioorg. Med. Chem. Lett. 1996;6:1323–1326.
- Kafarsky P, Lejczak B. Phosphorous, Sulfur, Silicon Relat. Elem. 1991;63:193–215. For comprehensive review, see:
- McLeod DA, Brinkworth RI, Ashley JA, Janda KD, Wirsching P. Bioorg. Med. Chem. Lett. 1991;1:653–658.
-
- Hirschmann R, Smith AB, III, Taylor CM, Benkovic PA, Taylor SD, Yager KM, Sprengler PA, Benkovic SJ. Science. 1994;265:234–237. - PubMed
- Allen MC, Fuhrer W, Tuck B, Wade R, Wood JM. J. Med. Chem. 1989;32:1652–1661. - PubMed
- Logusch EW, Walker DM, McDonald JF, Leo GC, Franz JE. J. Org. Chem. 1988;53:4069–4074.
- Giannousis PP, Bartlett PA. J. Med. Chem. 1987;30:1603–1609. - PubMed
-
- Emgenbroich M, Wulff G. Chem. Eur. J. 2003;9:4106–4117. - PubMed
- Sikorski JA, Miller MJ, Braccolino DS, Cleary DG, Corey SD, Font JL, Gruys KJ, Han CY, Lin K-C, Pajengraa PD, Ream JE, Schnur D, Shah A, Walker MC. Phosphorous, Sulfur, Silicon Relat. Elem. 1993;76:375–378.
-
- Senten K, Daniëls L, Var der Veken P, De Meester I, Lambeir A-M, Scharpé S, Haemers A, Augustyns K. J. Comb. Chem. 2003;5:336–344. - PubMed
- Stowasser B, Budt K-H, Jian-Qi L, Peyman A, Ruppert D. Tetrahedron Lett. 1992;33:6625–6628.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
