Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions
- PMID: 20872050
- PMCID: PMC3085450
- DOI: 10.1007/s11095-010-0277-5
Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions
Abstract
Purpose: Ultrasound can be used to release a therapeutic payload encapsulated within a perfluorocarbon (PFC) emulsion via acoustic droplet vaporization (ADV), a process whereby the PFC phase is vaporized and the agent is released. ADV-generated microbubbles have been previously used to selectively occlude blood vessels in vivo. The coupling of ADV-generated drug delivery and occlusion has therapeutically synergistic potentials.
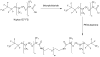
Methods: Micron-sized, water-in-PFC-in-water (W(1)/PFC/W(2)) emulsions were prepared in a two-step process using perfluoropentane (PFP) or perfluorohexane (PFH) as the PFC phase. Fluorescein or thrombin was contained in the W(1) phase.
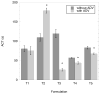
Results: Double emulsions containing fluorescein in the W(1) phase displayed a 5.7±1.4-fold and 8.2±1.3-fold increase in fluorescein mass flux, as measured using a Franz diffusion cell, after ADV for the PFP and PFH emulsions, respectively. Thrombin was stably retained in four out of five double emulsions. For three out of five formulations tested, the clotting time of whole blood decreased, in a statistically significant manner (p < 0.01), when incubated with thrombin-loaded emulsions exposed to ultrasound compared to emulsions not exposed to ultrasound.
Conclusions: ADV can be used to spatially and temporally control the delivery of water-soluble compounds formulated in PFC double emulsions. Thrombin release could extend the duration of ADV-generated, microbubble occlusions.
Figures




References
-
- Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. - PubMed
-
- Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci. 2007;32(8):962–990.
-
- Unger EC, Porter T, Culp W, LaBell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56(9):1291–1314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

