SH3BP2 mutations potentiate osteoclastogenesis via PLCγ
- PMID: 20872577
- PMCID: PMC2948751
- DOI: 10.1002/jor.21164
SH3BP2 mutations potentiate osteoclastogenesis via PLCγ
Abstract
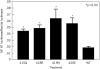
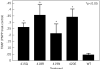
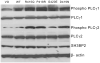
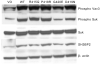
To determine the mechanism for the increased osteoclastogenesis in the jaw of cherubism patients with SH3BP2 mutations we evaluated the effect of mutant compared to wild-type SH3BP2 on activation of osteoclast signaling pathways. Indeed mutant forms of SH3BP2 do induce greater osteoclastogenesis. Heterozygous activating mutations in exon 9 of SH3BP2 have been found in most patients with cherubism, an unusual genetic syndrome characterized by excessive remodeling of the mandible and maxilla due to spontaneous and excessive osteoclastic bone resorption. Here we have investigated the functional consequences of SH3BP2 mutations on sRANKL-induced osteoclastogenesis in RAW 264.7 pre-osteoclast cells. sRANKL-stimulated RAW 264.7 cells were transfected with wild-type or mutant SH3BP2 plasmids. NFAT-luciferase and tartrate resistant acid phosphatase (TRAP), a marker of osteoclastic differentiation, levels were evaluated. Western immunoblots were also performed to determine phosphorylation of key proteins involved in the PI-PLC pathway leading to NFATc1 translocation. Our results indicate that forced expression of mutant forms of SH3BP2, found in cherubism patients, in RAW 264.7 cells induce greater NFAT activity and greater expression of TRAP than forced expression of wild-type SH3BP2. These findings indicate that missense SH3BP2 mutations cause a gain of protein function. Moreover, over expression of SH3BP2 in RAW 264.7 cells potentiates sRANKL-stimulated phosphorylation of PLCγ1 and PLCγ2. Our studies demonstrate that cherubism is due to gain-of-function mutations in SH3BP2 that stimulate RANKL-induced activation of PLCγ. The consequent activation of calcineurin and NFAT proteins induces the excessive osteoclastic phenotype of cherubism.
© 2010 Orthopaedic Research Society.
Figures

Similar articles
-
The role of SH3BP2 in the pathophysiology of cherubism.Orphanet J Rare Dis. 2012 May 24;7 Suppl 1(Suppl 1):S5. doi: 10.1186/1750-1172-7-S1-S5. Epub 2012 May 24. Orphanet J Rare Dis. 2012. PMID: 22640988 Free PMC article. Review.
-
SH3BP2 is an activator of NFAT activity and osteoclastogenesis.Biochem Biophys Res Commun. 2008 Jul 11;371(4):644-8. doi: 10.1016/j.bbrc.2008.04.080. Epub 2008 Apr 25. Biochem Biophys Res Commun. 2008. PMID: 18440306 Free PMC article.
-
SH3BP2 cherubism mutation potentiates TNF-α-induced osteoclastogenesis via NFATc1 and TNF-α-mediated inflammatory bone loss.J Bone Miner Res. 2014 Dec;29(12):2618-35. doi: 10.1002/jbmr.2295. J Bone Miner Res. 2014. PMID: 24916406 Free PMC article.
-
Decreased SH3BP2 inhibits osteoclast differentiation and function.J Orthop Res. 2011 Oct;29(10):1521-7. doi: 10.1002/jor.21408. Epub 2011 Mar 29. J Orthop Res. 2011. PMID: 21448930 Free PMC article.
-
A c.1244G>A (p.Arg415Gln) mutation in SH3BP2 gene causes cherubism in a Turkish family: report of a family with review of the literature.Med Oral Patol Oral Cir Bucal. 2014 Jul 1;19(4):e340-4. doi: 10.4317/medoral.19496. Med Oral Patol Oral Cir Bucal. 2014. PMID: 24608212 Free PMC article. Review.
Cited by
-
Phospholipases of mineralization competent cells and matrix vesicles: roles in physiological and pathological mineralizations.Int J Mol Sci. 2013 Mar 1;14(3):5036-129. doi: 10.3390/ijms14035036. Int J Mol Sci. 2013. PMID: 23455471 Free PMC article.
-
Tankyrase (PARP5) Inhibition Induces Bone Loss through Accumulation of Its Substrate SH3BP2.Cells. 2019 Feb 22;8(2):195. doi: 10.3390/cells8020195. Cells. 2019. PMID: 30813388 Free PMC article. Review.
-
Cloning and characterization of the human SH3BP2 promoter.Biochem Biophys Res Commun. 2012 Aug 17;425(1):25-32. doi: 10.1016/j.bbrc.2012.07.043. Epub 2012 Jul 17. Biochem Biophys Res Commun. 2012. PMID: 22820184 Free PMC article.
-
PLCG2 variants in cherubism.J Allergy Clin Immunol. 2024 Dec;154(6):1554-1558. doi: 10.1016/j.jaci.2024.08.016. Epub 2024 Aug 27. J Allergy Clin Immunol. 2024. PMID: 39197752
-
The role of SH3BP2 in the pathophysiology of cherubism.Orphanet J Rare Dis. 2012 May 24;7 Suppl 1(Suppl 1):S5. doi: 10.1186/1750-1172-7-S1-S5. Epub 2012 May 24. Orphanet J Rare Dis. 2012. PMID: 22640988 Free PMC article. Review.
References
-
- Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med. 2005;83:170–179. - PubMed
-
- Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. - PubMed
-
- Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E, et al. Increased Myeloid Cell Responses to M-CSF and RANKL Cause Bone Loss and Inflammation in SH3BP2 “Cherubism” Mice. Cell. 2007;128:71–83. - PubMed
-
- Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

