Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells
- PMID: 20872592
- PMCID: PMC3046385
- DOI: 10.1002/jor.21156
Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells
Abstract
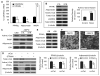
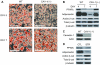
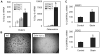
Regulation of skeletal remodeling appears to influence the differentiation of multipotent mesenchymal stem cells (MSC) resident in the bone marrow. As murine marrow cultures are contaminated with hematopoietic cells, they are problematic for studying direct effects of mechanical input. Here we use a modified technique to isolate marrow-derived MSC (mdMSC) from adult mice, yielding a population able to differentiate into adipogenic and osteogenic phenotypes that is devoid of hematopoietic cells. In pure mdMSC populations, a daily strain regimen inhibited adipogenic differentiation, suppressing expression of PPARγ and adiponectin. Strain increased β-catenin and inhibition of adipogenesis required this effect. Under osteogenic conditions, strain activated β-catenin signaling and increased expression of WISP1 and COX2. mdMSC were also generated from mice lacking caveolin-1, a protein known to sequester β-catenin: caveolin-1((-/-)) mdMSC exhibited retarded differentiation along both adipogenic and osteogenic lineages but retained mechanical responses that involved β-catenin activation. Interestingly, caveolin-1((-/-)) mdMSC failed to express bone sialoprotein and did not form mineralized nodules. In summary, mdMSC from adult mice respond to both soluble factors and mechanical input, with mechanical activation of β-catenin influencing phenotype. As such, these cells offer a useful model for studies of direct mechanical regulation of MSC differentiation and function.
© 2010 Orthopaedic Research Society.
Figures


References
-
- Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. - PubMed
-
- Armstrong VJ, Muzylak M, Sunters A, et al. Wnt/beta -catenin signaling is a component of osteoblastic bone cells’ early responses to load-bearing, and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. - PubMed
-
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. WNT/beta -catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

