Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis
- PMID: 20872832
- PMCID: PMC3012155
- DOI: 10.1002/ibd.21469
Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis
Abstract
Background: Intestinal epithelial expression of antioxidants and nuclear factor kappa B (NF-κB) contribute to mucosal barrier integrity and epithelial homeostasis, two key events in the pathogenesis of inflammatory bowel disease (IBD). Genetic restoration of intestinal epithelial prohibitin 1 (PHB) levels during experimental colitis reduces the severity of disease through sustained epithelial antioxidant expression and reduced NF-κB activation. To determine the therapeutic potential of restoring epithelial PHB during experimental colitis in mice, we assessed two methods of PHB colonic mucosal delivery: adenovirus-directed administration by enema and poly(lactic acid) nanoparticle (NPs) delivery by gavage.
Methods: As a proof-of-principle to demonstrate the therapeutic efficacy of PHB, we utilized adenovirus-directed administration by enema. Second, we used NPs-based colonic delivery of biologically active PHB to demonstrate therapeutic use for human IBD. Colitis was induced by oral administration of dextran sodium sulfate (DSS) in water for 6-7 days. Wildtype mice receiving normal tap water served as controls.
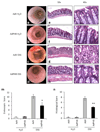
Results: Both methods of delivery resulted in increased levels of PHB in the surface epithelial cells of the colon and reduced severity of DSS-induced colitis in mice as measured by body weight loss, clinical score, myeloperoxidase activity, proinflammatory cytokine expression, histological score, and protein carbonyl content.
Conclusions: This is the first study to show oral delivery of a biologically active protein by NPs encapsulated in hydrogel to the colon. Here we show that therapeutic delivery of PHB to the colon reduces the severity of DSS-induced colitis in mice. PHB may represent a novel therapeutic target in IBD.
Copyright © 2010 Crohn's & Colitis Foundation of America, Inc.
Conflict of interest statement
Conflicts of Interest: The authors disclose no conflicts.
Figures






Similar articles
-
Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice.Gastroenterology. 2009 Jul;137(1):199-208, 208.e1-6. doi: 10.1053/j.gastro.2009.03.033. Epub 2009 Mar 25. Gastroenterology. 2009. PMID: 19327358 Free PMC article.
-
Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression.Mol Biol Cell. 2009 Oct;20(20):4412-23. doi: 10.1091/mbc.e09-05-0361. Epub 2009 Aug 26. Mol Biol Cell. 2009. PMID: 19710421 Free PMC article.
-
Nrf2 is not required for epithelial prohibitin-dependent attenuation of experimental colitis.Am J Physiol Gastrointest Liver Physiol. 2013 May 15;304(10):G885-96. doi: 10.1152/ajpgi.00327.2012. Epub 2013 Mar 14. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23494124 Free PMC article.
-
Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action.Pharmacol Res. 2012 Jul;66(1):66-79. doi: 10.1016/j.phrs.2012.03.013. Epub 2012 Mar 28. Pharmacol Res. 2012. PMID: 22475725
-
Isosteviol attenuates DSS-induced colitis by maintaining intestinal barrier function through PDK1/AKT/NF-κB signaling pathway.Int Immunopharmacol. 2023 Jan;114:109532. doi: 10.1016/j.intimp.2022.109532. Epub 2022 Dec 9. Int Immunopharmacol. 2023. PMID: 36508925
Cited by
-
CD98 siRNA-loaded nanoparticles decrease hepatic steatosis in mice.Dig Liver Dis. 2017 Feb;49(2):188-196. doi: 10.1016/j.dld.2016.11.008. Epub 2016 Nov 17. Dig Liver Dis. 2017. PMID: 27939923 Free PMC article.
-
Selection of nanobodies that block the enzymatic and cytotoxic activities of the binary Clostridium difficile toxin CDT.Sci Rep. 2015 Jan 19;5:7850. doi: 10.1038/srep07850. Sci Rep. 2015. PMID: 25597743 Free PMC article.
-
Nanomedicine in GI.Am J Physiol Gastrointest Liver Physiol. 2011 Mar;300(3):G371-83. doi: 10.1152/ajpgi.00466.2010. Epub 2010 Dec 9. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21148398 Free PMC article. Review.
-
Gut bacteria signaling to mitochondria in intestinal inflammation and cancer.Gut Microbes. 2020 May 3;11(3):285-304. doi: 10.1080/19490976.2019.1592421. Epub 2019 Mar 26. Gut Microbes. 2020. PMID: 30913966 Free PMC article. Review.
-
Prohibitin 1 modulates mitochondrial stress-related autophagy in human colonic epithelial cells.PLoS One. 2012;7(2):e31231. doi: 10.1371/journal.pone.0031231. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363587 Free PMC article.
References
-
- Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. - PubMed
-
- Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67–79. - PubMed
-
- Steinbrecher KA, Harmel-Laws E, Sitcheran R, et al. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–2599. - PubMed
-
- Artal-Sanz M, Tsang WY, Willems EM, et al. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem. 2003;278:32091–32099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK06411/DK/NIDDK NIH HHS/United States
- K01 DK085222/DK/NIDDK NIH HHS/United States
- R24-DK064399/DK/NIDDK NIH HHS/United States
- R01 HD057235/HD/NICHD NIH HHS/United States
- R01 DK071594/DK/NIDDK NIH HHS/United States
- R01 DK064711/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK061941/DK/NIDDK NIH HHS/United States
- R01-DK061941/DK/NIDDK NIH HHS/United States
- F32 DK076243/DK/NIDDK NIH HHS/United States
- R01-HD057235/HD/NICHD NIH HHS/United States
- K01-DK085222/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
