An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites
- PMID: 20872885
- PMCID: PMC3047707
- DOI: 10.1002/cyto.a.20972
An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites
Abstract
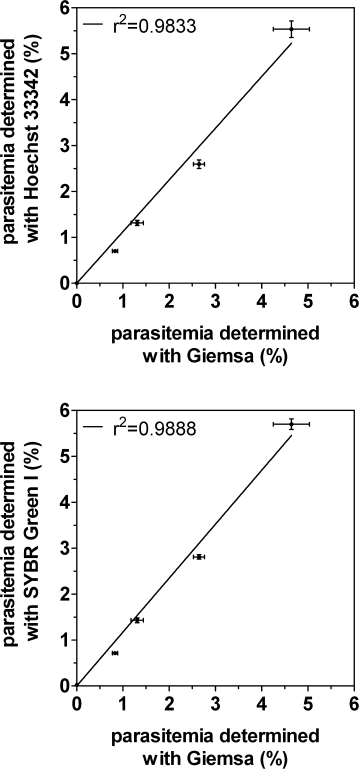
Plasmodium falciparum genotyping has recently undergone a revolution, and genome-wide genotype datasets are now being collected for large numbers of parasite isolates. By contrast, phenotyping technologies have lagged behind, with few high throughput phenotyping platforms available. Invasion of human erythrocytes by Plasmodium falciparum is a phenotype of particular interest because of its central role in parasite development. Invasion is a variable phenotype influenced by natural genetic variation in both the parasite and host and is governed by multiple overlapping and in some instances redundant parasite-erythrocyte interactions. To facilitate the scale-up of erythrocyte invasion phenotyping, we have developed a novel platform based on two-color flow cytometry that distinguishes parasite invasion from parasite growth. Target cells that had one or more receptors removed using enzymatic treatment were prelabeled with intracellular dyes CFDA-SE or DDAO-SE, incubated with P. falciparum parasites, and parasites that had invaded either labeled or unlabeled cells were detected with fluorescent DNA-intercalating dyes Hoechst 33342 or SYBR Green I. Neither cell label interfered with erythrocyte invasion, and the combination of cell and parasite dyes recapitulated known invasion phenotypes for three standard laboratory strains. Three different dye combinations with minimal overlap have been validated, meaning the same assay can be adapted to instruments harboring several different combinations of laser lines. The assay is sensitive, operates in a 96-well format, and can be used to quantitate the impact of natural or experimental genetic variation on erythrocyte invasion efficiency.
© 2010 International Society for Advancement of Cytometry.
Figures

Similar articles
-
A modified two-color flow cytometry assay to quantify in-vitro reinvasion and determine invasion phenotypes at low Plasmodium falciparum parasitemia.Exp Parasitol. 2020 Nov;218:107969. doi: 10.1016/j.exppara.2020.107969. Epub 2020 Aug 26. Exp Parasitol. 2020. PMID: 32858043
-
Enumeration of the Invasion Efficiency of Plasmodium falciparum In Vitro in Four Different Red Blood Cell Populations Using a Three-Color Flow Cytometry-Based Method.Cytometry A. 2019 Jul;95(7):737-745. doi: 10.1002/cyto.a.23750. Epub 2019 Mar 29. Cytometry A. 2019. PMID: 30924603
-
A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum.Am J Hematol. 2010 Apr;85(4):234-7. doi: 10.1002/ajh.21642. Am J Hematol. 2010. PMID: 20196166 Free PMC article.
-
Malaria Vaccine Development: Focusing Field Erythrocyte Invasion Studies on Phenotypic Diversity: The West African Merozoite Invasion Network (WAMIN).Trends Parasitol. 2016 Apr;32(4):274-283. doi: 10.1016/j.pt.2015.11.009. Epub 2015 Dec 23. Trends Parasitol. 2016. PMID: 26725306 Free PMC article. Review.
-
Cytoskeletal and membrane remodelling during malaria parasite invasion of the human erythrocyte.Br J Haematol. 2011 Sep;154(6):680-9. doi: 10.1111/j.1365-2141.2011.08766.x. Epub 2011 Jul 1. Br J Haematol. 2011. PMID: 21718279 Review.
Cited by
-
Comparative analysis of asexual and sexual stage Plasmodium falciparum development in different red blood cell types.Malar J. 2020 Jun 5;19(1):200. doi: 10.1186/s12936-020-03275-9. Malar J. 2020. PMID: 32503587 Free PMC article.
-
Normocyte-binding protein required for human erythrocyte invasion by the zoonotic malaria parasite Plasmodium knowlesi.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7231-6. doi: 10.1073/pnas.1522469113. Epub 2016 Jun 14. Proc Natl Acad Sci U S A. 2016. PMID: 27303038 Free PMC article.
-
Plasmodium Reproduction, Cell Size, and Transcription: How to Cope With Increasing DNA Content?Front Cell Infect Microbiol. 2021 Apr 9;11:660679. doi: 10.3389/fcimb.2021.660679. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33898332 Free PMC article.
-
An in vitro erythrocyte preference assay reveals that Plasmodium falciparum parasites prefer Type O over Type A erythrocytes.Sci Rep. 2018 May 25;8(1):8133. doi: 10.1038/s41598-018-26559-2. Sci Rep. 2018. PMID: 29802282 Free PMC article.
-
Variation in Plasmodium falciparum erythrocyte invasion phenotypes and merozoite ligand gene expression across different populations in areas of malaria endemicity.Infect Immun. 2015 Jun;83(6):2575-82. doi: 10.1128/IAI.03009-14. Epub 2015 Apr 13. Infect Immun. 2015. PMID: 25870227 Free PMC article.
References
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
-
- Gomez-Escobar N, Amambua-Ngwa A, Walther M, Okebe J, Ebonyi A, Conway DJ. Erythrocyte invasion and merozoite ligand gene expression in severe and mild Plasmodium falciparum malaria. J Infect Dis. 2010;201:444–452. - PubMed
-
- Bei AK, Membi CD, Rayner JC, Mubi M, Ngasala B, Sultan AA, Premji Z, Duraisingh MT. Variant merozoite protein expression is associated with erythrocyte invasion phenotypes in Plasmodium falciparum isolates from Tanzania. Mol Biochem Parasitol. 2007;153:66–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

