Diastereo- and enantioselective copper-catalyzed intramolecular carboamination of alkenes for the synthesis of hexahydro-1H-benz[f]indoles
- PMID: 20873817
- PMCID: PMC2966542
- DOI: 10.1021/ol102233g
Diastereo- and enantioselective copper-catalyzed intramolecular carboamination of alkenes for the synthesis of hexahydro-1H-benz[f]indoles
Abstract
A new method for the enantioselective synthesis of hexahydro-1H-benz[f]indoles is described. This copper-catalyzed enantioselective intramolecular alkene carboamination process can install vicinal tertiary and quaternary carbon stereocenters with high levels of diastereo- and enantioselectivity. The C-C bond-forming component of the reaction constitutes a C-H functionalization and no electronic activation of the aryl ring that undergoes addition is required. A known 5-HT(1A) receptor antagonist was synthesized efficiently using this method.
Figures

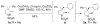

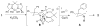

References
-
-
Reviews: Wolfe JP. Eur J Org Chem. 2007:571.Wolfe JP. Synlett. 2008:2913.Chemler SR. Org & Biomol Chem. 2009;7:3009.
-
-
-
Recent palladium-catalyzed carboaminations/carbonylations: Tsujihara T, Shinohara T, Takenaka K, Takizawa S, Onitsuka K, Hatanaka M, Sasai H. J Org Chem. 2009;74:9274.Mai DN, Wolfe JP. J Am Chem Soc. 2010;132:12157.He W, Yip KT, Zhu NY, Yang D. Org Lett. 2009;11:5626.Scarborough CC, Stahl SS. Org Lett. 2006;8:3251.Houlden CE, Bailey CD, Ford JG, Gagne MR, Lloyd-Jones GC, Booker-Milburn KI. J Am Chem Soc. 2008;130:10066.Scarborough CC, Bergant A, Sazama GT, Guzei IA, Spencer LC, Stahl SS. Tetrahedron. 2009;65:5084.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. J Am Chem Soc. 2009;131:9488.A gold-catalyzed carboamination: Zhang G, Cui L, Wang Y, Zhang L. J Am Chem Soc. 2010;132:1474.
-
-
-
Copper-catalyzed carboaminations: Zeng W, Chemler SR. J Am Chem Soc. 2007;129:12948.Sherman ES, Chemler SR. Adv Synth & Catal. 2009;351:467.Cu(II)-promoted carboaminations: Sherman ES, Fuller PH, Kasi D, Chemler SR. J Org Chem. 2007;72:3896.Fuller PH, Chemler SR. Org Lett. 2007;9:5477.
-
-
- Lin CH, Haadsma-Svensson SR, Phillips G, Lahti RA, McCall RB, Piercey MF, Schreur PJKD, Von Voigtlander PF, Smith MW, Chidester CG. J Med Chem. 1993;36:1069. - PubMed
-
- Park HJ, Lee HJ, Min HY, Chung HJ, Suh ME, Park-Choo HY, Kim C, Kim HJ, Seo EK, Lee SK. Eur J Pharm. 2005;527:31. and references therein. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

