Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation
- PMID: 20873820
- PMCID: PMC3199963
- DOI: 10.1021/ja1046186
Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation
Abstract
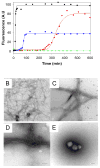
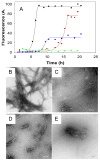
Amyloid formation plays a role in over 25 human disorders. A range of strategies have been applied to the problem of developing inhibitors of amyloid formation, but unfortunately, many inhibitors are effective only in molar excess and typically either lengthen the time to the onset of amyloid formation, (the lag time), while having modest effects on the total amount of amyloid fibrils produced, or decrease the amount of amyloid without significantly reducing the lag time. We demonstrate a general strategy whereby two moderate inhibitors of amyloid formation can be rationally selected via kinetic assays and combined in trans to yield a highly effective inhibitor which dramatically delays the time to the appearance of amyloid and drastically reduces the total amount of amyloid formed. A key feature is that the selection of the components of the mixture is based on their effect on the time course of amyloid formation rather than on just the amount of amyloid produced. The approach is validated using inhibitors of amyloid formation by islet amyloid polypeptide, the causative agent of amyloid formation in type 2 diabetes and the Alzheimer's disease Aβ peptide.
Figures
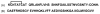
Similar articles
-
Coffee components inhibit amyloid formation of human islet amyloid polypeptide in vitro: possible link between coffee consumption and diabetes mellitus.J Agric Food Chem. 2011 Dec 28;59(24):13147-55. doi: 10.1021/jf201702h. Epub 2011 Nov 21. J Agric Food Chem. 2011. PMID: 22059381
-
Molecular characterization of the hetero-assembly of β-amyloid peptide with islet amyloid polypeptide.Curr Pharm Des. 2014;20(8):1182-91. doi: 10.2174/13816128113199990064. Curr Pharm Des. 2014. PMID: 23713771
-
Ultraeffective Inhibition of Amyloid Fibril Assembly by Nanobody-Gold Nanoparticle Conjugates.Bioconjug Chem. 2019 Jan 16;30(1):29-33. doi: 10.1021/acs.bioconjchem.8b00797. Epub 2018 Dec 26. Bioconjug Chem. 2019. PMID: 30585717
-
Molecular physiology of the islet amyloid polypeptide (IAPP)/amylin gene in man, rat, and transgenic mice.J Cell Biochem. 1994;55 Suppl:39-53. doi: 10.1002/jcb.240550006. J Cell Biochem. 1994. PMID: 7929617 Review.
-
Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes?Diabetologia. 2004 Feb;47(2):157-69. doi: 10.1007/s00125-003-1304-4. Epub 2004 Jan 13. Diabetologia. 2004. PMID: 14722650 Review.
Cited by
-
Ion mobility spectrometry-mass spectrometry defines the oligomeric intermediates in amylin amyloid formation and the mode of action of inhibitors.J Am Chem Soc. 2014 Jan 15;136(2):660-70. doi: 10.1021/ja406831n. Epub 2013 Dec 30. J Am Chem Soc. 2014. PMID: 24372466 Free PMC article.
-
Islet amyloid polypeptide toxicity and membrane interactions.Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19279-84. doi: 10.1073/pnas.1305517110. Epub 2013 Nov 11. Proc Natl Acad Sci U S A. 2013. PMID: 24218607 Free PMC article.
-
Analysis of the ability of pramlintide to inhibit amyloid formation by human islet amyloid polypeptide reveals a balance between optimal recognition and reduced amyloidogenicity.Biochemistry. 2015 Nov 10;54(44):6704-11. doi: 10.1021/acs.biochem.5b00567. Epub 2015 Oct 30. Biochemistry. 2015. PMID: 26407043 Free PMC article.
-
Conformationally restricted short peptides inhibit human islet amyloid polypeptide (hIAPP) fibrillization.Chem Commun (Camb). 2013 Apr 4;49(26):2688-90. doi: 10.1039/c3cc38982k. Chem Commun (Camb). 2013. PMID: 23435449 Free PMC article.
-
Structural similarities and differences between amyloidogenic and non-amyloidogenic islet amyloid polypeptide (IAPP) sequences and implications for the dual physiological and pathological activities of these peptides.PLoS Comput Biol. 2013;9(8):e1003211. doi: 10.1371/journal.pcbi.1003211. Epub 2013 Aug 29. PLoS Comput Biol. 2013. PMID: 24009497 Free PMC article.
References
-
- Selkoe DJ. Nat Cell Biol. 2004;6:1054. - PubMed
-
- Caughey B, Lansbury PT. Annu Rev Neurosci. 2003;26:267. - PubMed
-
- Chiti F, Dobson CM. Annu Rev Biochem. 2006;75:333. - PubMed
-
- Cohen FE, Kelly JW. Nature. 2003;426:905. - PubMed
-
- Chen SM, Berthelier V, Hamilton JB, O’Nuallain B, Wetzel R. Biochemistry. 2002;41:7391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

