Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery
- PMID: 20873958
- PMCID: PMC2996815
- DOI: 10.1089/neu.2010.1456
Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery
Abstract
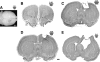
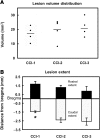
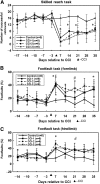
We report the results of controlled cortical impact (CCI) centered on the caudal forelimb area (CFA) of rat motor cortex to determine the feasibility of examining cortical plasticity in a spared cortical motor area (rostral forelimb area, RFA). We compared the effects of three CCI parameter sets (groups CCI-1, CCI-2, and CCI-3) that differed in impactor surface shape, size, and location, on behavioral recovery and RFA structural and functional integrity. Forelimb deficits in the limb contralateral to the injury were evident in all three CCI groups assessed by skilled reach and footfault tasks that persisted throughout the 35-day post-CCI assessment period. Nissl-stained coronal sections revealed that the RFA was structurally intact. Intracortical microstimulation experiments conducted at 7 weeks post-CCI demonstrated that RFA was functionally viable. However, the size of the forelimb representation decreased significantly in CCI-1 compared to the control group. Subdivided into component movement categories, there was a significant group effect for proximal forelimb movements. The RFA area reduction and reorganization are discussed in relation to possible diaschisis, and to compensatory functional behavior, respectively. Also, an inverse correlation between the anterior extent of the lesion and the size of the RFA was identified and is discussed in relation to corticocortical connectivity. The results suggest that CCI can be applied to rat CFA while sparing RFA. This CCI model can contribute to our understanding of neural plasticity in premotor cortex as a substrate for functional motor recovery.
Figures


References
-
- Adkins D.L. Jones T.A. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci. Lett. 2005;380:214–218. - PubMed
-
- Alaverdashvili M. Foroud A. Lim D.H. Whishaw I.Q. “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behav. Brain Res. 2008;188:281–290. - PubMed
-
- Alaverdashvili M. Lim D.H. Whishaw I. Q. No improvement by amphetamine on learned non-use, attempts, success or movement in skilled reaching by the rat after motor cortex stroke. Eur. J. Neurosci. 2007;25:3442–3452. - PubMed
-
- Barth T.M. Jones T.A. Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav. Brain Res. 1990;39:73–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

