Long-term safety of mometasone furoate/formoterol combination for treatment of patients with persistent asthma
- PMID: 20874458
- PMCID: PMC2993043
- DOI: 10.3109/02770903.2010.514634
Long-term safety of mometasone furoate/formoterol combination for treatment of patients with persistent asthma
Erratum in
- J Asthma. 2011 Feb;48(1):114
Abstract
Objective: The combination of inhaled corticosteroid (ICS) and long-acting β₂-agonist is recommended for treatment of patients with persistent asthma inadequately controlled on ICS monotherapy. This study was conducted to evaluate the long-term safety of mometasone furoate/formoterol (MF/F) administered through metered-dose inhaler (MDI) in patients with persistent asthma previously on medium- to high-dose ICS.
Methods: This was a 52-week, randomized, multicenter, parallel-group, open-label, evaluator-blinded study. At baseline, 404 patients (aged ≥12 years) were stratified according to their previous ICS dose (medium or high), then randomized 2:1 to receive twice-daily treatment of MF/F (200/10 or 400/10 μg) or fluticasone propionate/salmeterol (FP/S; 250/50 or 500/50 μg). The primary endpoint was the number and percentage of patients reporting any adverse event (AE). Additional safety evaluations included plasma cortisol 24-hour area under the curve (AUC(0-24 h)) and ocular changes. Pulmonary function, asthma symptoms, and use of rescue medication were monitored.
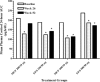
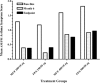
Results: The incidence of ≥1 treatment-emergent AE was similar across treatment groups (MF/F 200/10 μg, 77.3% [n= 109]; FP/S 250/50 μg, 82.4% [n= 56]; MF/F 400/10 μg, 79.2% [n= 103]; FP/S 500/50 μg, 76.9% [n= 50]). Rates of treatment-related AEs were also similar across treatment groups (MF/F 200/10 μg, 28.4%; FP/S 250/50 μg, 23.5%; MF/F 400/10 μg, 23.1%; FP/S 500/50 μg, 20.0%). Headache (3.7%) and dysphonia (2.7%) were the most common treatment-related AEs overall. The nature and frequency of AEs and the decreases in plasma cortisol AUC(0-24 h) observed with MF/F treatment were similar to those observed with FP/S treatment. Ocular events were rare (2-6% overall incidence among treatment groups); in particular, no posterior subcapsular cataracts were reported. Only three patients discontinued the study because of treatment-related ocular AEs (two for lens disorders in the MF/F 400/10 μg group; one for reduced visual acuity in the FP/S 250/50 μg group) and no asthma-related deaths occurred. Furthermore, MF/F showed numerical improvement in lung function and clinical benefits by reducing asthma symptoms and rescue medication use.
Conclusions: One-year treatment with the new combination therapies - twice-daily MF/F-MDI 200/10 and 400/10 μg - is safe and well tolerated in patients with persistent asthma.
Figures

References
-
- Murphy KR. Asthma: versatile treatment for a variable disease. J Asthma. 2005;42:149–157. - PubMed
-
- Bernstein DI. ABCs of asthma. Clin Cornerstone. 2008;8:9–25. - PubMed
-
- Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. - PubMed
-
- Global Initiative for Asthma. Global strategy for asthma manage ment and prevention. Available at: http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=1561. Accessed July 29, 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
