Thrombin-induced endothelin-1 synthesis and secretion in retinal pigment epithelial cells is rho kinase dependent
- PMID: 20874501
- PMCID: PMC2956378
- DOI: 10.1089/jop.2010.0072
Thrombin-induced endothelin-1 synthesis and secretion in retinal pigment epithelial cells is rho kinase dependent
Abstract
Purpose: The retinal pigment epithelium (RPE) is a major source for endothelin-1 (ET-1), a potent vasoactive peptide, at the outer blood–retinal barrier. Factors that regulate ET-1 synthesis at this site may help identify its normal function and its role in pathologic states accompanying retinal injury. Thrombin is one such factor that might act on the RPE after injury and breakdown of the blood–retinal barrier. The present study was conducted to identify signaling intermediates in thrombin-induced ET-1 synthesis and secretion in primary human RPE (hRPE) and transformed RPE cells (ARPE-19) and a possible pharmacological strategy to block excess release of ET-1.
Methods: Cultured hRPE cells were treated with different concentrations of thrombin and thrombin receptor agonists, and a time course to measure levels of preproET-1 (ppET-1) mRNA and secreted mature ET-1 was performed. Levels of secondary messengers [Ca²+]i and RhoA were measured and pharmacologically inhibited to determine how receptor-mediated thrombin activity lead to changes in ET-1 levels.
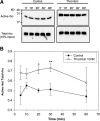
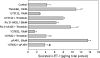
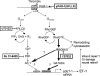
Results: Thrombin primarily acts via the protease-activated receptor-1 (PAR-1) subtype in RPE to induce ET-1 synthesis. Thrombin and other receptor agonists increased both [Ca²+]<]i and active RhoA. PAR-1-dependent rho/Rho kinase activation led to increase in ppET-1 mRNA and mature ET-1 secretion.
Conclusions: Transient intracellular calcium mobilization and protein kinase C activation by thrombin play a minor role, if any, in ET-1 synthesis in RPE. Instead, rho/Rho kinase activation after PAR-1 stimulation strongly increased ppET-1 mRNA and ET-1 secretion in hRPE cells.
Figures




Similar articles
-
Endothelin-1 synthesis and secretion in human retinal pigment epithelial cells (ARPE-19): differential regulation by cholinergics and TNF-alpha.Invest Ophthalmol Vis Sci. 2003 Nov;44(11):4885-94. doi: 10.1167/iovs.03-0387. Invest Ophthalmol Vis Sci. 2003. PMID: 14578413
-
Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways.J Biol Chem. 2009 Jul 31;284(31):20638-48. doi: 10.1074/jbc.M109.004762. Epub 2009 May 12. J Biol Chem. 2009. PMID: 19439413 Free PMC article.
-
Thrombin promotes actin stress fiber formation in RPE through Rho/ROCK-mediated MLC phosphorylation.J Cell Physiol. 2011 Feb;226(2):414-23. doi: 10.1002/jcp.22347. J Cell Physiol. 2011. PMID: 20672289
-
Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-beta2 in human retinal pigment epithelial cells.Exp Eye Res. 2007 Mar;84(3):464-72. doi: 10.1016/j.exer.2006.10.017. Epub 2007 Jan 10. Exp Eye Res. 2007. PMID: 17217948
-
Ocular immune privilege and retinal pigment epithelial cells.J Leukoc Biol. 2023 Mar 1;113(3):288-304. doi: 10.1093/jleuko/qiac016. J Leukoc Biol. 2023. PMID: 36805720 Review.
Cited by
-
Thrombin-Induced Calpain Activation Promotes Protease-Activated Receptor 1 Internalization.Int J Cell Biol. 2017;2017:1908310. doi: 10.1155/2017/1908310. Epub 2017 Nov 9. Int J Cell Biol. 2017. PMID: 29250115 Free PMC article.
-
Expression of Pro-Angiogenic Markers Is Enhanced by Blue Light in Human RPE Cells.Antioxidants (Basel). 2020 Nov 20;9(11):1154. doi: 10.3390/antiox9111154. Antioxidants (Basel). 2020. PMID: 33233546 Free PMC article.
-
Protease-activated receptors in vascular smooth muscle cells: a bridge between thrombo-inflammation and vascular remodelling.Cell Commun Signal. 2025 Jan 31;23(1):57. doi: 10.1186/s12964-025-02066-6. Cell Commun Signal. 2025. PMID: 39891111 Free PMC article. Review.
-
Inhalation of particulate matter containing environmentally persistent free radicals induces endothelial dysfunction mediated via AhR activation at the air-blood interface.Toxicol Sci. 2024 May 28;199(2):246-260. doi: 10.1093/toxsci/kfae007. Toxicol Sci. 2024. PMID: 38310335 Free PMC article.
References
-
- Marmor M. New York: Oxford University Press; 1998. Structure, Function, and Disease of the Retinal Pigment Epithelium; pp. 3–9.
-
- Campochiaro P.A. Growth factors in the retinal pigment epithelium and retina. In: Marmor M.F., editor; Wolfensberger T.J., editor. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 459–477.
-
- MacCumber M.W. Jampel H.D. Snyder S.H. Ocular effects of the endothelins. Abundant peptides in the eye. Arch. Ophthalmol. 1991;109:705–709. - PubMed
-
- Wollensak G. Schaefer H.E. Ihling C. An immunohistochemical study of endothelin-1 in the human eye. Curr. Eye Res. 1998;17:541–545. - PubMed
-
- Ripodas A. de Juan J.A. Roldan-Pallares M., et al. Localisation of endothelin-1 mRNA expression and immunoreactivity in the retina and optic nerve from human and porcine eye. Evidence for endothelin-1 expression in astrocytes. Brain Res. 2001;912:137–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

