In vivo reflectance confocal microscopy of shave biopsy wounds: feasibility of intraoperative mapping of cancer margins
- PMID: 20874785
- PMCID: PMC2988110
- DOI: 10.1111/j.1365-2133.2010.10063.x
In vivo reflectance confocal microscopy of shave biopsy wounds: feasibility of intraoperative mapping of cancer margins
Abstract
Background: Reflectance confocal microscopy (RCM) images skin at cellular resolution and has shown utility for the diagnosis of nonmelanoma skin cancer in vivo. Topical application of aluminium chloride (AlCl(3)) enhances contrast in RCM images by brightening nuclei.
Objectives: To investigate feasibility of RCM imaging of shave biopsy wounds using AlCl(3) as a contrast agent.
Methods: AlCl(3) staining was optimized, in terms of concentration vs. immersion time, on excised tissue ex vivo. RCM imaging protocol was tested in patients undergoing shave biopsies. The RCM images were retrospectively analysed and compared with the corresponding histopathology.
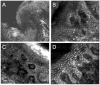
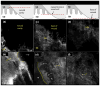
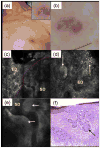
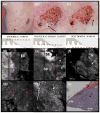
Results: For 35% AlCl(3) , routinely used for haemostasis in clinic, minimum immersion time was determined to be 1 min. We identified three consistent patterns of margins on RCM mosaic images by varying depth: epidermal margins, peripheral dermal margins, and deep dermal margins. Tumour islands of basal cell carcinoma were identified at peripheral or deep dermal margins, correlating on histopathology with aggregates of neoplastic basaloid cells. Atypical cobblestone or honeycomb patterns were identified at the epidermal margins in squamous cell carcinomas, correlating with a proliferation of atypical keratinocytes extending to biopsy margins.
Conclusions: RCM imaging of shave biopsy wounds is feasible and demonstrates the future possibility of intraoperative mapping in surgical wounds.
© 2010 The Authors. BJD © 2010 British Association of Dermatologists.
Conflict of interest statement
Figures



Similar articles
-
Peri-operative delineation of non-melanoma skin cancer margins in vivo with handheld reflectance confocal microscopy and video-mosaicking.J Eur Acad Dermatol Venereol. 2019 Jun;33(6):1084-1091. doi: 10.1111/jdv.15491. Epub 2019 Mar 15. J Eur Acad Dermatol Venereol. 2019. PMID: 30811707 Free PMC article.
-
In vivo reflectance confocal microscopy of Basal cell carcinoma: feasibility of preoperative mapping of cancer margins.Dermatol Surg. 2012 Dec;38(12):1945-50. doi: 10.1111/j.1524-4725.2012.02587.x. Epub 2012 Oct 5. Dermatol Surg. 2012. PMID: 23039159 Free PMC article.
-
Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: initial clinical experience.J Biomed Opt. 2015 Jun;20(6):61103. doi: 10.1117/1.JBO.20.6.061103. J Biomed Opt. 2015. PMID: 25706821 Free PMC article.
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
-
Through the looking glass: Basics and principles of reflectance confocal microscopy.J Am Acad Dermatol. 2015 Aug;73(2):276-84. doi: 10.1016/j.jaad.2015.04.047. Epub 2015 Jun 4. J Am Acad Dermatol. 2015. PMID: 26051696 Review.
Cited by
-
Performance of full-pupil line-scanning reflectance confocal microscopy in human skin and oral mucosa in vivo.Biomed Opt Express. 2011 Jul 1;2(7):2055-67. doi: 10.1364/BOE.2.002055. Epub 2011 Jun 27. Biomed Opt Express. 2011. PMID: 21750780 Free PMC article.
-
Confocal microscopy to guide erbium:yttrium aluminum garnet laser ablation of basal cell carcinoma: an ex vivo feasibility study.J Biomed Opt. 2013 Sep;18(9):095001. doi: 10.1117/1.JBO.18.9.095001. J Biomed Opt. 2013. PMID: 24045654 Free PMC article.
-
Peri-operative delineation of non-melanoma skin cancer margins in vivo with handheld reflectance confocal microscopy and video-mosaicking.J Eur Acad Dermatol Venereol. 2019 Jun;33(6):1084-1091. doi: 10.1111/jdv.15491. Epub 2019 Mar 15. J Eur Acad Dermatol Venereol. 2019. PMID: 30811707 Free PMC article.
-
Optical techniques for the noninvasive diagnosis of skin cancer.J Cancer Res Clin Oncol. 2013 Jul;139(7):1083-104. doi: 10.1007/s00432-013-1423-3. Epub 2013 Apr 4. J Cancer Res Clin Oncol. 2013. PMID: 23552870 Free PMC article. Review.
-
Rapid screening of cancer margins in tissue with multimodal confocal microscopy.J Surg Res. 2012 Dec;178(2):533-8. doi: 10.1016/j.jss.2012.05.059. Epub 2012 Jun 7. J Surg Res. 2012. PMID: 22721570 Free PMC article.
References
-
- Bialy TL, Whalen J, Veledar E, et al. Mohs micrographic surgery vs traditional surgical excision: a cost comparison analysis. Arch Dermatol. 2004;140:736–42. - PubMed
-
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. - PubMed
-
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. - PubMed
-
- Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. - PubMed
-
- Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

