Germ layer differentiation during early hindgut and cloaca formation in rabbit and pig embryos
- PMID: 20874819
- PMCID: PMC3039179
- DOI: 10.1111/j.1469-7580.2010.01303.x
Germ layer differentiation during early hindgut and cloaca formation in rabbit and pig embryos
Abstract
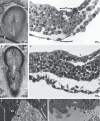
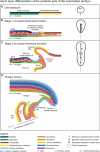
Relative to recent advances in understanding molecular requirements for endoderm differentiation, the dynamics of germ layer morphology and the topographical distribution of molecular factors involved in endoderm formation at the caudal pole of the embryonic disc are still poorly defined. To discover common principles of mammalian germ layer development, pig and rabbit embryos at late gastrulation and early neurulation stages were analysed as species with a human-like embryonic disc morphology, using correlative light and electron microscopy. Close intercellular contact but no direct structural evidence of endoderm formation such as mesenchymal-epithelial transition between posterior primitive streak mesoderm and the emerging posterior endoderm were found. However, a two-step process closely related to posterior germ layer differentiation emerged for the formation of the cloacal membrane: (i) a continuous mesoderm layer and numerous patches of electron-dense flocculent extracellular matrix mark the prospective region of cloacal membrane formation; and (ii) mesoderm cells and all extracellular matrix including the basement membrane are lost locally and close intercellular contact between the endoderm and ectoderm is established. The latter process involves single cells at first and then gradually spreads to form a longitudinally oriented seam-like cloacal membrane. These gradual changes were found from gastrulation to early somite stages in the pig, whereas they were found from early somite to mid-somite stages in the rabbit; in both species cloacal membrane formation is complete prior to secondary neurulation. The results highlight the structural requirements for endoderm formation during development of the hindgut and suggest new mechanisms for the pathogenesis of common urogenital and anorectal malformations.
© 2010 The Authors. Journal of Anatomy © 2010 Anatomical Society of Great Britain and Ireland.
Figures



References
-
- Blum M, Andre P, Muders K, et al. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation. 2007;75:133–146. - PubMed
-
- Burtscher I, Lickert H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development. 2009;136:1029–1038. - PubMed
-
- Charrier J, Teillet M, Lapointe F, et al. Defining subregions of Hensen's node essential for caudalward movement, midline development and cell survival. Development. 1999;126:4771–4783. - PubMed
-
- Downs KM. Systematic localization of oct-3/4 to the gastrulating mouse conceptus suggests manifold roles in mammalian development. Dev Dyn. 2008;237:464–475. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

