A role for phospholipase A2 activity in membrane tubule formation and TGN trafficking
- PMID: 20874826
- PMCID: PMC4906538
- DOI: 10.1111/j.1600-0854.2010.01115.x
A role for phospholipase A2 activity in membrane tubule formation and TGN trafficking
Abstract
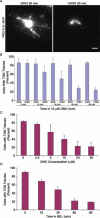
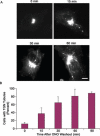
We have investigated the role of phospholipase A(2) (PLA(2) ) enzymes in generating membrane tubules at the trans-Golgi network (TGN). Constitutive TGN membrane tubules and those induced by over-expressing kinase dead protein kinase D were inhibited by the PLA(2) inhibitors ONO-RS-082 (ONO) and bromoenol lactone. These antagonists also inhibited secretory delivery of both soluble and transmembrane cargoes. Finally, use of the reversible antagonist ONO and time-lapse imaging revealed for the first time that PLA(2) antagonists inhibit the initiation of membrane tubule formation at the TGN. Thus, PLA(2) enzymes appear to have an important role in the earliest steps of membrane tubule formation at the TGN, which are utilized for membrane trafficking.
© 2010 John Wiley & Sons A/S.
Figures



Similar articles
-
Assembly of an intact Golgi complex requires phospholipase A2 (PLA2) activity, membrane tubules, and dynein-mediated microtubule transport.Biochem Biophys Res Commun. 2009 Nov 20;389(3):473-7. doi: 10.1016/j.bbrc.2009.08.173. Epub 2009 Sep 9. Biochem Biophys Res Commun. 2009. PMID: 19747452 Free PMC article.
-
Arachidonyltrifluoromethy ketone, a phospholipase A(2) antagonist, induces dispersal of both Golgi stack- and trans Golgi network-resident proteins throughout the cytoplasm.Biochem Biophys Res Commun. 2001 Feb 23;281(2):582-8. doi: 10.1006/bbrc.2001.4381. Biochem Biophys Res Commun. 2001. PMID: 11181087
-
Cytoplasmic phospholipase A2 antagonists inhibit multiple endocytic membrane trafficking pathways.Biochem Biophys Res Commun. 2009 Oct 30;388(4):695-9. doi: 10.1016/j.bbrc.2009.08.067. Epub 2009 Aug 18. Biochem Biophys Res Commun. 2009. PMID: 19695219 Free PMC article.
-
The formation of TGN-to-plasma-membrane transport carriers.Annu Rev Cell Dev Biol. 2006;22:439-55. doi: 10.1146/annurev.cellbio.21.012704.133126. Annu Rev Cell Dev Biol. 2006. PMID: 16824007 Review.
-
Phosphoinositides and membrane traffic at the trans-Golgi network.Biochem Soc Symp. 2005;(72):31-8. doi: 10.1042/bss0720031. Biochem Soc Symp. 2005. PMID: 15649127 Review.
Cited by
-
Gβ1γ2 activates phospholipase A2-dependent Golgi membrane tubule formation.Front Cell Dev Biol. 2014 Feb 28;2(4):0004. doi: 10.3389/fcell.2014.00004. Front Cell Dev Biol. 2014. PMID: 25019068 Free PMC article.
-
Role of phospholipase A(2) in retrograde transport of ricin.Toxins (Basel). 2011 Sep;3(9):1203-19. doi: 10.3390/toxins3091203. Epub 2011 Sep 23. Toxins (Basel). 2011. PMID: 22069763 Free PMC article.
-
Morpho-functional architecture of the Golgi complex of neuroendocrine cells.Front Endocrinol (Lausanne). 2013 Mar 28;4:41. doi: 10.3389/fendo.2013.00041. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23543640 Free PMC article.
-
Possible Involvement of Intracellular Calcium-Independent Phospholipase A2 in the Release of Secretory Phospholipases from Mast Cells-Increased Expression in Ileal Mast Cells of Crohn's Disease.Cells. 2019 Jul 3;8(7):672. doi: 10.3390/cells8070672. Cells. 2019. PMID: 31277247 Free PMC article.
-
Impaired Transferrin Receptor Palmitoylation and Recycling in Neurodegeneration with Brain Iron Accumulation.Am J Hum Genet. 2018 Feb 1;102(2):266-277. doi: 10.1016/j.ajhg.2018.01.003. Am J Hum Genet. 2018. PMID: 29395073 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

