The initial experience of electronic brachytherapy for the treatment of non-melanoma skin cancer
- PMID: 20875139
- PMCID: PMC2957390
- DOI: 10.1186/1748-717X-5-87
The initial experience of electronic brachytherapy for the treatment of non-melanoma skin cancer
Abstract
Background: Millions of people are diagnosed with non-melanoma skin cancers (NMSC) worldwide each year. While surgical approaches are the standard treatment, some patients are appropriate candidates for radiation therapy for NMSC. High dose rate (HDR) brachytherapy using surface applicators has shown efficacy in the treatment of NMSC and shortens the radiation treatment schedule by using a condensed hypofractionated approach. An electronic brachytherapy (EBT) system permits treatment of NMSC without the use of a radioactive isotope.
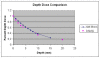
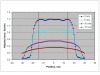
Methods: Data were collected retrospectively from patients treated from July 2009 through March 2010. Pre-treatment biopsy was performed to confirm a malignant cutaneous diagnosis. A CT scan was performed to assess lesion depth for treatment planning, and an appropriate size of surface applicator was selected to provide an acceptable margin. An HDR EBT system delivered a dose of 40.0 Gy in eight fractions twice weekly with 48 hours between fractions, prescribed to a depth of 3-7 mm. Treatment feasibility, acute safety, efficacy outcomes, and cosmetic results were assessed.
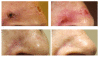
Results: Thirty-seven patients (mean age 72.5 years) with 44 cutaneous malignancies were treated. Of 44 lesions treated, 39 (89%) were T1, 1 (2%) Tis, 1 (2%) T2, and 3 (7%) lesions were recurrent. Lesion locations included the nose for 16 lesions (36.4%), ear 5 (11%), scalp 5 (11%), face 14 (32%), and an extremity for 4 (9%). Median follow-up was 4.1 months. No severe toxicities occurred. Cosmesis ratings were good to excellent for 100% of the lesions at follow-up.
Conclusions: The early outcomes of EBT for the treatment of NMSC appear to show acceptable acute safety and favorable cosmetic outcomes. Using a hypofractionated approach, EBT provides a convenient treatment schedule.
Figures


Similar articles
-
Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer.Pract Radiat Oncol. 2015 Nov-Dec;5(6):e659-64. doi: 10.1016/j.prro.2015.07.002. Epub 2015 Jul 17. Pract Radiat Oncol. 2015. PMID: 26432680
-
Custom mold applicator high-dose-rate brachytherapy for nonmelanoma skin cancer-An analysis of 273 lesions.Brachytherapy. 2018 May-Jun;17(3):601-608. doi: 10.1016/j.brachy.2018.01.002. Epub 2018 Feb 15. Brachytherapy. 2018. PMID: 29398593
-
Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year.Brachytherapy. 2013 Mar-Apr;12(2):134-40. doi: 10.1016/j.brachy.2012.08.003. Epub 2013 Jan 9. Brachytherapy. 2013. PMID: 23312675 Clinical Trial.
-
Review: the reemergence of brachytherapy as treatment for non-melanoma skin cancer.J Dermatolog Treat. 2018 Mar;29(2):170-175. doi: 10.1080/09546634.2017.1341617. Epub 2017 Jun 30. J Dermatolog Treat. 2018. PMID: 28604229 Review.
-
Aspects of dosimetry and clinical practice of skin brachytherapy: The American Brachytherapy Society working group report.Brachytherapy. 2015 Nov-Dec;14(6):840-58. doi: 10.1016/j.brachy.2015.06.005. Epub 2015 Aug 28. Brachytherapy. 2015. PMID: 26319367 Review.
Cited by
-
Electronic brachytherapy management of atypical fibroxanthoma: report of 8 lesions.J Contemp Brachytherapy. 2017 Apr;9(2):158-160. doi: 10.5114/jcb.2017.65454. Epub 2017 Jan 25. J Contemp Brachytherapy. 2017. PMID: 28533805 Free PMC article.
-
Incorporation of Electronic Brachytherapy for Skin Cancer into a Community Dermatology Practice.J Clin Aesthet Dermatol. 2015 Nov;8(11):28-32. J Clin Aesthet Dermatol. 2015. PMID: 26705437 Free PMC article.
-
Does ultrasound measurement improve the accuracy of electronic brachytherapy in the treatment of superficial non-melanomatous skin cancer?J Contemp Brachytherapy. 2017 Feb;9(1):14-19. doi: 10.5114/jcb.2017.65476. Epub 2017 Jan 26. J Contemp Brachytherapy. 2017. PMID: 28344599 Free PMC article.
-
Novel treatment options for nonmelanoma skin cancer: focus on electronic brachytherapy.Med Devices (Auckl). 2015 Nov 26;8:493-502. doi: 10.2147/MDER.S61585. eCollection 2015. Med Devices (Auckl). 2015. PMID: 26648763 Free PMC article. Review.
-
Electronic brachytherapy--current status and future directions.Br J Radiol. 2015 May;88(1049):20150002. doi: 10.1259/bjr.20150002. Epub 2015 Mar 6. Br J Radiol. 2015. PMID: 25748070 Free PMC article. Review.
References
-
- Skin cancer - UK incidence statistics. Cancer Research UK. http://info.cancerresearchuk.org/cancerstats/types/skin/index.htm Accessed June 30, 2010.
-
- Skin Cancer Net. Schaumberg, Illinois: American Academy of Dermatology; http://www.skincarephysicians.com/skincancernet/whatis.html Accessed June 17, 2010.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

