Stacking and energetic contribution of aromatic islands at the binding interface of antibody proteins
- PMID: 20875152
- PMCID: PMC2946779
- DOI: 10.1186/1745-7580-6-S1-S1
Stacking and energetic contribution of aromatic islands at the binding interface of antibody proteins
Abstract
Background: The enrichment and importance of some aromatic residues, such as Tyr and Trp, have been widely noticed at the binding interfaces of antibodies from many experimental and statistical results, some of which were even identified as "hot spots" contributing significantly greater to the binding affinity than other amino acids. However, how these aromatic residues influence the immune binding still deserves further investigation. A large-scale examination was done regarding the local spatial environment around the interfacial Tyr or Trp residues. Energetic contribution of these Tyr and Trp residues to the binding affinity was then studied regarding 82 representative antibody interfaces covering 509 immune complexes from the PDB database and IMGT/3Dstructure-DB.
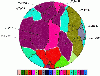
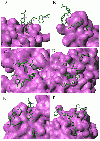
Results: The connectivity analysis of interfacial residues showed that Tyr and Trp tended to cluster into the spatial Aromatic Islands (AI) rather than being distributed randomly at the antibody interfaces. Out of 82 antibody-antigen complexes, 72% (59) interfaces were found to contain AI with more than 3 aromatic residues. The statistical test against an empirical distribution indicated that the existence of AI was significant in about 60% representative antibody interfaces. Secondly, the loss of solvent accessible surface area (SASA) for side chains of aromatic residues between actually crowded state and independent state was nicely correlated with the AI size increasing in a linearly positive way which indicated that the aromatic side chains in AI tended to take a compact and ordered stacking conformation at the interfaces. Interestingly, the SASA loss of AI was also correlated roughly with the averaged gap of binding free energy between the theoretical and experimental data for immune complexes.
Conclusions: The results of our study revealed the wide existence and statistical significance of "Aromatic Island" (AI) composed of the spatially clustered Tyr and Trp residues at the antibody interfaces. The regular arrangement and stacking of aromatic side chains in AI could probably produce extra cooperative effects to the binding affinity which was firstly observed through the large-scale data analysis. The finding in this work not only provides insights into the functional role of aromatic residues in the antibody-antigen interaction, but also may facilitate the antibody engineering and potential clinical applications.
Figures


Similar articles
-
Analyses on clustering of the conserved residues at protein-RNA interfaces and its application in binding site identification.BMC Bioinformatics. 2020 Feb 17;21(1):57. doi: 10.1186/s12859-020-3398-9. BMC Bioinformatics. 2020. PMID: 32066366 Free PMC article.
-
Empirical estimation of the energetic contribution of individual interface residues in structures of protein-protein complexes.J Comput Aided Mol Des. 2009 Sep;23(9):645-54. doi: 10.1007/s10822-009-9282-3. Epub 2009 May 29. J Comput Aided Mol Des. 2009. PMID: 19479323
-
Analysis of antibody A6 binding to the extracellular interferon gamma receptor alpha-chain by alanine-scanning mutagenesis and random mutagenesis with phage display.Biochemistry. 2000 Dec 26;39(51):15674-85. doi: 10.1021/bi000838z. Biochemistry. 2000. PMID: 11123892
-
Structure, function and properties of antibody binding sites.J Mol Biol. 1991 Jan 5;217(1):133-51. doi: 10.1016/0022-2836(91)90617-f. J Mol Biol. 1991. PMID: 1988675 Review.
-
The aromatic stacking interactions between proteins and their macromolecular ligands.Curr Protein Pept Sci. 2015;16(6):502-12. doi: 10.2174/138920371606150702131516. Curr Protein Pept Sci. 2015. PMID: 26138814 Review.
Cited by
-
Polyclonal antibody responses to HIV Env immunogens resolved using cryoEM.Nat Commun. 2021 Aug 10;12(1):4817. doi: 10.1038/s41467-021-25087-4. Nat Commun. 2021. PMID: 34376662 Free PMC article.
-
PInteract: Detecting Aromatic-Involving Motifs in Proteins and Protein-Nucleic Acid Complexes.Biomolecules. 2025 Aug 21;15(8):1204. doi: 10.3390/biom15081204. Biomolecules. 2025. PMID: 40867649 Free PMC article.
-
Molecular determinants for antibody binding on group 1 house dust mite allergens.J Biol Chem. 2012 Mar 2;287(10):7388-98. doi: 10.1074/jbc.M111.311159. Epub 2011 Dec 30. J Biol Chem. 2012. PMID: 22210776 Free PMC article.
-
A new protein-ligand binding sites prediction method based on the integration of protein sequence conservation information.BMC Bioinformatics. 2011 Dec 14;12 Suppl 14(Suppl 14):S9. doi: 10.1186/1471-2105-12-S14-S9. BMC Bioinformatics. 2011. PMID: 22373099 Free PMC article.
-
Epitope predictions indicate the presence of two distinct types of epitope-antibody-reactivities determined by epitope profiling of intravenous immunoglobulins.PLoS One. 2013 Nov 11;8(11):e78605. doi: 10.1371/journal.pone.0078605. eCollection 2013. PLoS One. 2013. PMID: 24244326 Free PMC article.
References
-
- Kabat EA, Wu TT, Bilofsky H. Unusual distributions of amino acids in complementarity-determining (hypervariable) segments of heavy and light chains of immunoglobulins and their possible roles in specificity of antibody-combining sites. J. Biol. Chem. 1977;252:6609–6616. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
