VDAC3 has differing mitochondrial functions in two types of striated muscles
- PMID: 20875390
- PMCID: PMC2998388
- DOI: 10.1016/j.bbabio.2010.09.007
VDAC3 has differing mitochondrial functions in two types of striated muscles
Abstract
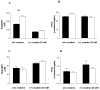
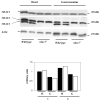
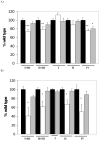
Voltage-dependent anion channel (VDAC) is an abundant mitochondrial outer membrane protein. In mammals, three VDAC isoforms have been characterized. We have previously reported alterations in the function of mitochondria when assessed in situ in different muscle types in VDAC1 deficient mice (Anflous et al., 2001). In the present report we extend the study to VDAC3 deficient muscles and measure the respiratory enzyme activity in both VDAC1 and VDAC3 deficient muscles. While in the heart the absence of VDAC3 causes a decrease in the apparent affinity of in situ mitochondria for ADP, in the gastrocnemius, a mixed glycolytic/oxidative muscle, the affinity of in situ mitochondria for ADP remains unchanged. The absence of VDAC1 causes multiple defects in respiratory complex activities in both types of muscle. However, in VDAC3 deficient mice the defect is restricted to the heart and only to complex IV. These functional alterations correlate with structural aberrations of mitochondria. These results demonstrate that, unlike VDAC1, there is muscle-type specificity for VDAC3 function and therefore in vivo these two isoforms may fulfill different physiologic functions.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


References
-
- Brown GC, Lakin-Thomas PL, Brand MD. Control of respiration and oxidative phosphorylation in isolated rat liver cells. Eur J Biochem. 1990;192:355–362. - PubMed
-
- Gellerich FN, Bohnensack R, Kunz W. Role of the mitochondrial outer membrane in dynamic compartmentation of adenine nucleotides. In: Azzi A, Nalecz KA, Nalecz MJ, Wojtczak L, editors. The Anion Carriers of the Mitochondrial Membranes. Springer Verlag; Berlin Heidelberg: 1989. pp. 349–359.
-
- Saks VA, Belikova YO, Kuznetsov AV. In vivo regulation of mitochondrial respiration in cardiomyocytes: specific restriction for intracellular diffusion for ADP. Biochim Biophys Acta. 1991;1074:302–311. - PubMed
-
- Saks VA, Vasil'eva E, Belikova YO, Kuznetsov AV, Lyapina S, Petrova L, Perov NA. Retarded diffusion of ADP in cardiomyocytes: possible role of mitochondrial outer membrane and creatine kinase in cellular regulation of oxidative phosphorylation. Biochim Biophys Acta. 1993;1144:134–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

