The transcription factor Spn1 regulates gene expression via a highly conserved novel structural motif
- PMID: 20875428
- PMCID: PMC2993574
- DOI: 10.1016/j.jmb.2010.09.040
The transcription factor Spn1 regulates gene expression via a highly conserved novel structural motif
Abstract
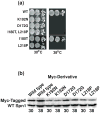
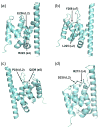
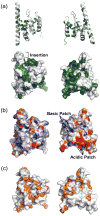
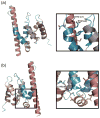
Spn1/Iws1 plays essential roles in the regulation of gene expression by RNA polymerase II (RNAPII), and it is highly conserved in organisms ranging from yeast to humans. Spn1 physically and/or genetically interacts with RNAPII, TBP (TATA-binding protein), TFIIS (transcription factor IIS), and a number of chromatin remodeling factors (Swi/Snf and Spt6). The central domain of Spn1 (residues 141-305 out of 410) is necessary and sufficient for performing the essential functions of SPN1 in yeast cells. Here, we report the high-resolution (1.85 Å) crystal structure of the conserved central domain of Saccharomyces cerevisiae Spn1. The central domain is composed of eight α-helices in a right-handed superhelical arrangement and exhibits structural similarity to domain I of TFIIS. A unique structural feature of Spn1 is a highly conserved loop, which defines one side of a pronounced cavity. The loop and the other residues forming the cavity are highly conserved at the amino acid level among all Spn1 family members, suggesting that this is a signature motif for Spn1 orthologs. The locations and the molecular characterization of temperature-sensitive mutations in Spn1 indicate that the cavity is a key attribute of Spn1 that is critical for its regulatory functions during RNAPII-mediated transcriptional activity.
Copyright © 2010. Published by Elsevier Ltd.
Figures



Similar articles
-
Spn1 regulates the recruitment of Spt6 and the Swi/Snf complex during transcriptional activation by RNA polymerase II.Mol Cell Biol. 2008 Feb;28(4):1393-403. doi: 10.1128/MCB.01733-07. Epub 2007 Dec 17. Mol Cell Biol. 2008. PMID: 18086892 Free PMC article.
-
Structure and biological importance of the Spn1-Spt6 interaction, and its regulatory role in nucleosome binding.Mol Cell. 2010 Dec 10;40(5):725-35. doi: 10.1016/j.molcel.2010.11.014. Epub 2010 Nov 25. Mol Cell. 2010. PMID: 21094070 Free PMC article.
-
SPN1, a conserved gene identified by suppression of a postrecruitment-defective yeast TATA-binding protein mutant.Genetics. 2002 Dec;162(4):1605-16. doi: 10.1093/genetics/162.4.1605. Genetics. 2002. PMID: 12524336 Free PMC article.
-
A brief overview of the Swi1 prion-[SWI+].FEMS Yeast Res. 2018 Sep 1;18(6):foy061. doi: 10.1093/femsyr/foy061. FEMS Yeast Res. 2018. PMID: 29905794 Free PMC article. Review.
-
Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review.
Cited by
-
Genome Instability Is Promoted by the Chromatin-Binding Protein Spn1 in Saccharomyces cerevisiae.Genetics. 2018 Dec;210(4):1227-1237. doi: 10.1534/genetics.118.301600. Epub 2018 Oct 9. Genetics. 2018. PMID: 30301740 Free PMC article.
-
The structure of an Iws1/Spt6 complex reveals an interaction domain conserved in TFIIS, Elongin A and Med26.EMBO J. 2010 Dec 1;29(23):3979-91. doi: 10.1038/emboj.2010.272. Epub 2010 Nov 5. EMBO J. 2010. PMID: 21057455 Free PMC article.
-
GWAS Based on RNA-Seq SNPs and High-Throughput Phenotyping Combined with Climatic Data Highlights the Reservoir of Valuable Genetic Diversity in Regional Tomato Landraces.Genes (Basel). 2020 Nov 23;11(11):1387. doi: 10.3390/genes11111387. Genes (Basel). 2020. PMID: 33238469 Free PMC article.
-
Analytical Ultracentrifugation (AUC): An Overview of the Application of Fluorescence and Absorbance AUC to the Study of Biological Macromolecules.Curr Protoc Mol Biol. 2020 Dec;133(1):e131. doi: 10.1002/cpmb.131. Curr Protoc Mol Biol. 2020. PMID: 33351266 Free PMC article.
-
High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3- uptake is associated with changes in histone methylation.Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13329-34. doi: 10.1073/pnas.1017863108. Epub 2011 Jul 25. Proc Natl Acad Sci U S A. 2011. PMID: 21788519 Free PMC article.
References
-
- Saunders A, Core LJ, Lis JT. Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol. 2006;7:557–67. - PubMed
-
- Svejstrup JD. The RNA polymerase II transcription cycle: cycling through chromatin. Biochim Biophys Acta. 2004;1677:64–73. - PubMed
-
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

