Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants
- PMID: 20876112
- PMCID: PMC2955100
- DOI: 10.1073/pnas.0911359107
Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants
Abstract
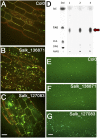
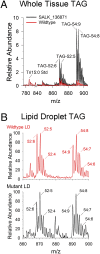
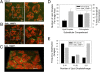
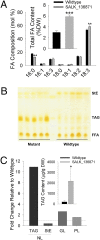
CGI-58 is the defective gene in the human neutral lipid storage disease called Chanarin-Dorfman syndrome. This disorder causes intracellular lipid droplets to accumulate in nonadipose tissues, such as skin and blood cells. Here, disruption of the homologous CGI-58 gene in Arabidopsis thaliana resulted in the accumulation of neutral lipid droplets in mature leaves. Mass spectroscopy of isolated lipid droplets from cgi-58 loss-of-function mutants showed they contain triacylglycerols with common leaf-specific fatty acids. Leaves of mature cgi-58 plants exhibited a marked increase in absolute triacylglycerol levels, more than 10-fold higher than in wild-type plants. Lipid levels in the oil-storing seeds of cgi-58 loss-of-function plants were unchanged, and unlike mutations in β-oxidation, the cgi-58 seeds germinated and grew normally, requiring no rescue with sucrose. We conclude that the participation of CGI-58 in neutral lipid homeostasis of nonfat-storing tissues is similar, although not identical, between plant and animal species. This unique insight may have implications for designing a new generation of technologies that enhance the neutral lipid content and composition of crop plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Graham IA. Seed storage oil mobilization. Annu Rev Plant Biol. 2008;59:115–142. - PubMed
-
- Cahoon EB, et al. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: Solving bottlenecks in fatty acid flux. Curr Opin Plant Biol. 2007;10:236–244. - PubMed
-
- Damude HG, Kinney AJ. Engineering oilseeds to produce nutritional fatty acids. Physiol Plant. 2008;132(1):1–10. - PubMed
-
- Dyer JM, Stymne S, Green AG, Carlsson AS. High-value oils from plants. Plant J. 2008;54:640–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

