O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart
- PMID: 20876116
- PMCID: PMC2955091
- DOI: 10.1073/pnas.1001907107
O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart
Abstract
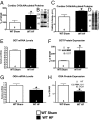
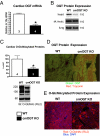
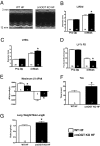
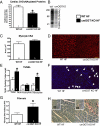
The failing heart is subject to elevated metabolic demands, adverse remodeling, chronic apoptosis, and ventricular dysfunction. The interplay among such pathologic changes is largely unknown. Several laboratories have identified a unique posttranslational modification that may have significant effects on cardiovascular function. The O-linked β-N-acetylglucosamine (O-GlcNAc) posttranslational modification (O-GlcNAcylation) integrates glucose metabolism with intracellular protein activity and localization. Because O-GlcNAc is derived from glucose, we hypothesized that altered O-GlcNAcylation would occur during heart failure and figure prominently in its pathophysiology. After 5 d of coronary ligation in WT mice, cardiac O-GlcNAc transferase (OGT; which adds O-GlcNAc to proteins) and levels of O-GlcNAcylation were significantly (P < 0.05) elevated in the surviving remote myocardium. We used inducible, cardiac myocyte-specific Cre recombinase transgenic mice crossed with loxP-flanked OGT mice to genetically delete cardiomyocyte OGT (cmOGT KO) and ascertain its role in the failing heart. After tamoxifen induction, cardiac O-GlcNAcylation of proteins and OGT levels were significantly reduced compared with WT, but not in other tissues. WT and cardiomyocyte OGT KO mice underwent nonreperfused coronary ligation and were followed for 4 wk. Although OGT deletion caused no functional change in sham-operated mice, OGT deletion in infarcted mice significantly exacerbated cardiac dysfunction compared with WT. These data provide keen insights into the pathophysiology of the failing heart and illuminate a previously unrecognized point of integration between metabolism and cardiac function in the failing heart.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

