Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons
- PMID: 20876196
- PMCID: PMC3008853
- DOI: 10.1113/jphysiol.2010.197012
Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons
Abstract
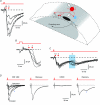
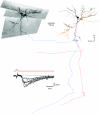
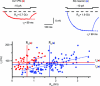
Spinal lamina I receives nociceptive primary afferent input to project through diverse ascending pathways, including the anterolateral tract (ALT). Large projection neurons (PNs) form only a few per cent of the cell population in this layer, and little is known about their local input from other lamina I neurons. We combined single-cell imaging in the isolated spinal cord, paired recordings, 3-D reconstructions of biocytin-labelled neurons and computer simulations to study the monosynaptic input to large ALT-PNs from neighbouring (somata separated by less than 80 μm) large lamina I neurons. All 11 connections identified were excitatory. We have found that an axon of a presynaptic neuron forms multiple synapses on an ALT-PN, and both Ca(2+)-permeable and Ca(2+)-impermeable AMPA receptors are involved in transmission. The monosynaptic EPSC latencies (1-12 ms) are determined by both post- and presynaptic factors. The postsynaptic delay, resulting from the electrotonic EPSC propagation in the dendrites of an ALT-PN, could be 4 ms at most. The presynaptic delay, caused by the spike propagation in a narrow highly branched axon of a local-circuit neuron, can be about 10 ms for neighbouring ALT-PNs and longer for more distant neurons. In many cases, the EPSPs evoked by release from a lamina I neuron were sufficient to elicit a spike in an ALT-PN. Our data show that ALT-PNs can receive input from both lamina I local-circuit neurons and other ALT-PNs. We suggest that lamina I is a functionally interconnected layer. The intralaminar network described here can amplify the overall output from the principal spinal nociceptive projection area.
Figures





References
-
- Bice TN, Beal JA. Quantitative and neurogenic analysis of the total population and subpopulations of neurons defined by axon projection in the superficial dorsal horn of the rat lumbar spinal cord. J Comp Neurol. 1997;388:550–564. - PubMed
-
- Cajal RyS. Histologie du systeme nerveux de l'homme et des vertebres. Paris: A. Maloine; 1909.
-
- Graham BA, Brichta AM, Callister RJ. Moving from an averaged to specific view of spinal cord pain processing circuits. J Neurophysiol. 2007;98:1057–1063. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

