Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter
- PMID: 20876197
- PMCID: PMC3008855
- DOI: 10.1113/jphysiol.2010.196618
Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter
Abstract
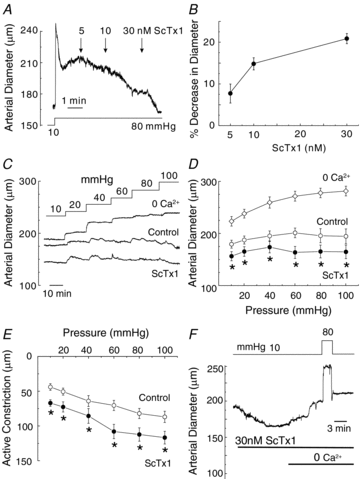
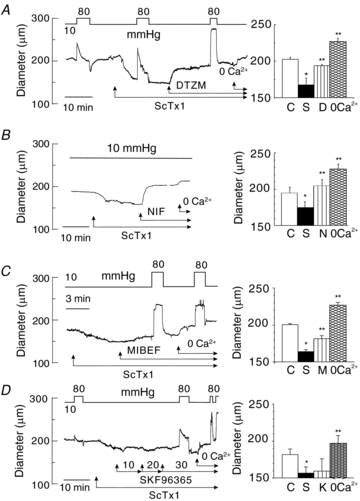
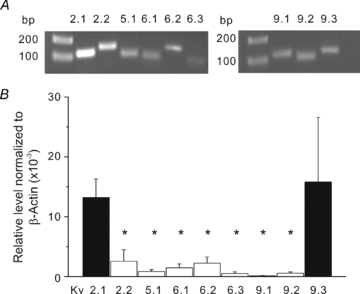
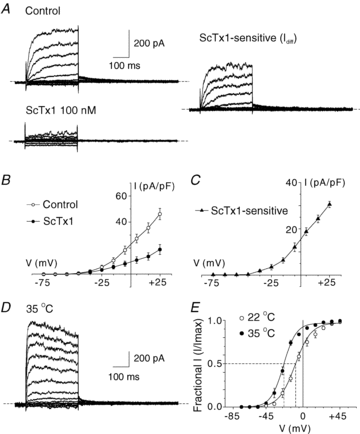
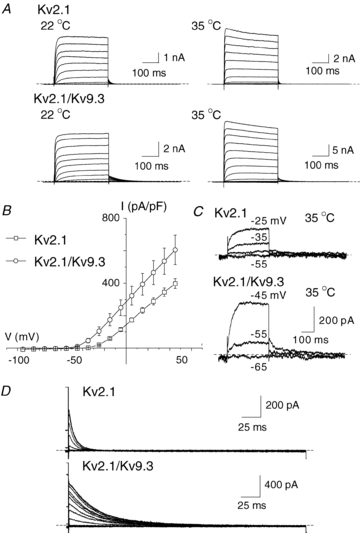
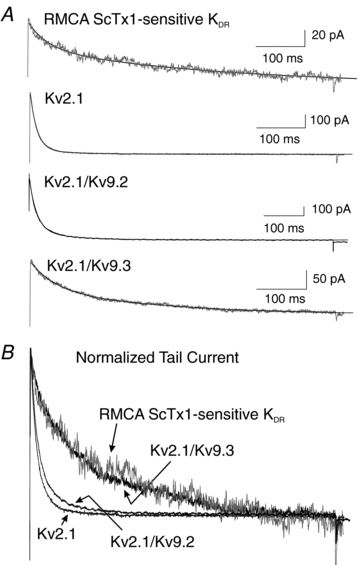
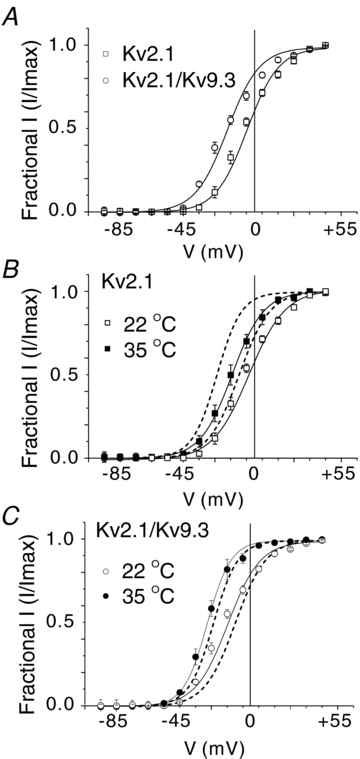
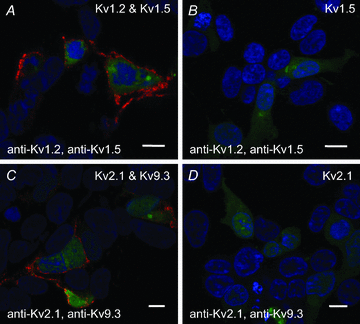
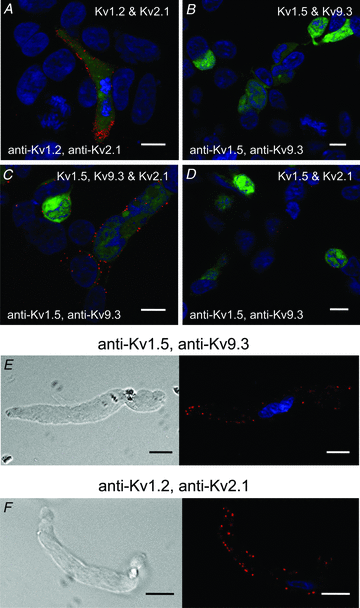
Cerebral vascular smooth muscle contractility plays a crucial role in controlling arterial diameter and, thereby, blood flow regulation in the brain. A number of K(+) channels have been suggested to contribute to the regulation of diameter by controlling smooth muscle membrane potential (E(m)) and Ca(2+) influx. Previous studies indicate that stromatoxin (ScTx1)-sensitive, Kv2-containing channels contribute to the control of cerebral arterial diameter at 80 mmHg, but their precise role and molecular composition were not determined. Here, we tested if Kv2 subunits associate with 'silent' subunits from the Kv5, Kv6, Kv8 or Kv9 subfamilies to form heterotetrameric channels that contribute to control of diameter of rat middle cerebral arteries (RMCAs) over a range of intraluminal pressure from 10 to 100 mmHg. The predominant mRNAs expressed by RMCAs encode Kv2.1 and Kv9.3 subunits. Co-localization of Kv2.1 and Kv9.3 proteins at the plasma membrane of dissociated single RMCA myocytes was detected by proximity ligation assay. ScTx1-sensitive native current of RMCA myocytes and Kv2.1/Kv9.3 currents exhibited functional identity based on the similarity of their deactivation kinetics and voltage dependence of activation that were distinct from those of homomultimeric Kv2.1 channels. ScTx1 treatment enhanced the myogenic response of pressurized RMCAs between 40 and 100 mmHg, but this toxin also caused constriction between 10 and 40 mmHg that was not previously observed following inhibition of large conductance Ca(2+)-activated K(+) (BK(Ca)) and Kv1 channels. Taken together, this study defines the molecular basis of Kv2-containing channels and contributes to our understanding of the functional significance of their expression in cerebral vasculature. Specifically, our findings provide the first evidence of heteromultimeric Kv2.1/Kv9.3 channel expression in RMCA myocytes and their distinct contribution to control of cerebral arterial diameter over a wider range of E(m) and transmural pressure than Kv1 or BK(Ca) channels owing to their negative range of voltage-dependent activation.
Figures
References
-
- Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. - PubMed
-
- Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–C356. - PubMed
-
- Belevych AE, Beck R, Tammaro P, Poston L, Smirnov SV. Developmental changes in the functional characteristics and expression of voltage-gated K+ channel currents in rat aortic myocytes. Cardiovasc Res. 2002;54:152–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

