Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine
- PMID: 20876203
- PMCID: PMC3008851
- DOI: 10.1113/jphysiol.2010.196824
Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine
Abstract
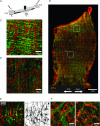
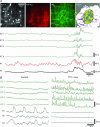
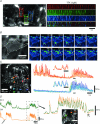
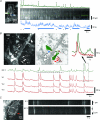
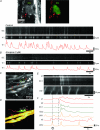
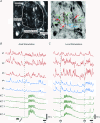
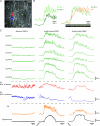
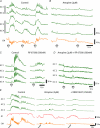
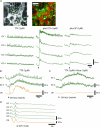
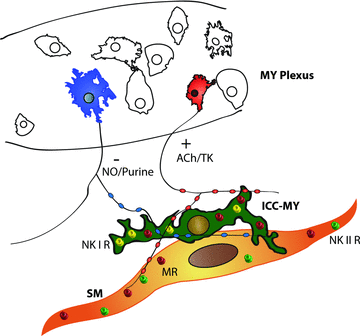
Colonic migrating motor complexes (CMMCs) are neurally mediated, cyclical contractile and electrical events, which typically propagate along the colon every 2-3 min in the mouse. We examined the interactions between myenteric neurons, interstitial cells of Cajal in the myenteric region (ICC-MY) and smooth muscle cells during CMMCs using Ca(2+) imaging. CMMCs occurred spontaneously or were evoked by stimulating the mucosa locally, or by brushing it at either end of the colon. Between CMMCs, most ICC-MY were often quiescent; their lack of activity was correlated with ongoing Ca(2+) transients in varicosities on the axons of presumably inhibitory motor neurons that were on or surrounded ICC-MY. Ca(2+) transients in other varicosities initiated intracellular Ca(2+) waves in adjacent ICC-MY, which were blocked by atropine, suggesting they were on the axons of excitatory motor neurons. Following TTX (1 μM), or blockade of inhibitory neurotransmission with N(ω)-nitro-L-arginine (L-NA, a NO synthesis inhibitor, 10 μM) and MRS 2500 (a P2Y(1) antagonist, 1 μM), ongoing spark/puff like activity and rhythmic intracellular Ca(2+) waves (38.1 ± 2.9 cycles min(-1)) were observed, yet this activity was uncoupled, even between ICC-MY in close apposition. During spontaneous or evoked CMMCs there was an increase in the frequency (62.9 ± 1.4 cycles min(-1)) and amplitude of Ca(2+) transients in ICC-MY and muscle, which often had synchronized activity. At the same time, activity in varicosites along excitatory and inhibitory motor nerve fibres increased and decreased respectively, leading to an overall excitation of ICC-MY. Atropine (1 μM) reduced the evoked responses in ICC-MY, and subsequent addition of an NK1 antagonist (RP 67580, 500 nM) completely blocked the responses to stimulation, as did applying these drugs in reverse order. An NKII antagonist (MEN 10,376, 500 nM) had no effect on the evoked responses in ICC-MY. Following TTX application, carbachol (1 μM), substance P (1 μM) and an NKI agonist (GR73632, 100 nM) produced the fast oscillations superimposed on a slow increase in Ca(2+) in ICC-MY, whereas SNP (an NO donor, 10 μM) abolished all activity in ICC-MY. In conclusion, ICC-MY, which are under tonic inhibition, are pacemakers whose activity can be synchronized by excitatory nerves to couple the longitudinal and circular muscles during the CMMC. ICC-MY receive excitatory input from motor neurons that release acetylcholine and tachykinins acting on muscarinic and NK1 receptors, respectively.
Figures

References
-
- Aranishi H, Kunisawa Y, Komuro T. Characterization of interstitial cells of Cajal in the subserosal layer of the guinea-pig colon. Cell Tissue Res. 2009;335:323–329. - PubMed
-
- Bayguinov PO, Hennig GW, Smith TK. Generation of complex neuronal behavior in a mammalian nervous system. Physiological News. 2010b in press.
-
- Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

