PECAM-targeted delivery of SOD inhibits endothelial inflammatory response
- PMID: 20876216
- PMCID: PMC3005426
- DOI: 10.1096/fj.10-169789
PECAM-targeted delivery of SOD inhibits endothelial inflammatory response
Abstract
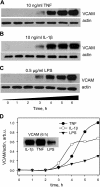
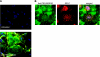
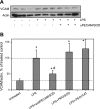
Elevated generation of reactive oxygen species (ROS) by endothelial enzymes, including NADPH-oxidase, is implicated in vascular oxidative stress and endothelial proinflammatory activation involving exposure of vascular cell adhesion molecule-1 (VCAM-1). Catalase and superoxide dismutase (SOD) conjugated with antibodies to platelet/endothelial cell adhesion molecule 1 (PECAM-1) bind specifically to endothelium and inhibit effects of corresponding ROS, H(2)O(2), and superoxide anion. In this study, anti-PECAM/SOD, but not anti-PECAM/catalase or nontargeted enzymes, including polyethylene glycol (PEG)-SOD, inhibited 2- to 3-fold VCAM expression caused by tumor necrosis factor (TNF), interleukin-1β, and lipopolysaccharide. Anti- PECAM/SOD, but not nontargeted counterparts, accumulated in vascular endothelium after intravenous injection, localized in endothelial endosomes, and inhibited by 70% lipopolysaccharide-caused VCAM-1 expression in mice. Anti-PECAM/SOD colocalized with EEA-1-positive endothelial vesicles and quenched ROS produced in response to TNF. Inhibitors of NADPH oxidase and anion channel ClC3 blocked TNF-induced VCAM expression, affirming that superoxide produced and transported by these proteins, respectively, mediates inflammatory signaling. Anti-PECAM/SOD abolished VCAM expression caused by poly(I:C)-induced activation of toll-like receptor 3 localized in intracellular vesicles. These results directly implicate endosomal influx of superoxide in endothelial inflammatory response and suggest that site-specific interception of this signal attained by targeted delivery of anti-PECAM/SOD into endothelial endosomes may have anti-inflammatory effects.
Figures
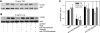

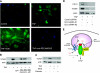
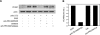
References
-
- Rhee S. G. (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 - PubMed
-
- Gow A. J., Farkouh C. R., Munson D. A., Posencheg M. A., Ischiropoulos H. (2004) Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L262–L268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

