The sperm nucleus: chromatin, RNA, and the nuclear matrix
- PMID: 20876223
- PMCID: PMC5358669
- DOI: 10.1530/REP-10-0322
The sperm nucleus: chromatin, RNA, and the nuclear matrix
Abstract
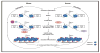
Within the sperm nucleus, the paternal genome remains functionally inert and protected following protamination. This is marked by a structural morphogenesis that is heralded by a striking reduction in nuclear volume. Despite these changes, both human and mouse spermatozoa maintain low levels of nucleosomes that appear non-randomly distributed throughout the genome. These regions may be necessary for organizing higher order genomic structure through interactions with the nuclear matrix. The promoters of this transcriptionally quiescent genome are differentially marked by modified histones that may poise downstream epigenetic effects. This notion is supported by increasing evidence that the embryo inherits these differing levels of chromatin organization. In concert with the suite of RNAs retained in the mature sperm, they may synergistically interact to direct early embryonic gene expression. Irrespective, these features reflect the transcriptional history of spermatogenic differentiation. As such, they may soon be utilized as clinical markers of male fertility. In this review, we explore and discuss how this may be orchestrated.
Figures
References
-
- Adenot PG, Mercier Y, Renard JP, Thompson EM. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development. 1997;124:4615–4625. - PubMed
-
- Adom JN, Richard-Foy H. A region immediately adjacent to the origin of replication of bovine papilloma virus type 1 interacts in vitro with the nuclear matrix. Biochem Biophys Res Commun. 1991;176:479–485. - PubMed
-
- Albrethsen J, Knol JC, Jimenez CR. Unravelling the nuclear matrix proteome. J Proteomics. 2009;72:71–81. - PubMed
-
- Amanai M, Brahmajosyula M, Perry AC. A restricted role for sperm-borne microRNAs in mammalian fertilization. Biol Reprod. 2006;75:877–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

