Comparison of the regulation, metabolic functions, and roles in virulence of the glyceraldehyde-3-phosphate dehydrogenase homologues gapA and gapB in Staphylococcus aureus
- PMID: 20876289
- PMCID: PMC2981307
- DOI: 10.1128/IAI.00762-10
Comparison of the regulation, metabolic functions, and roles in virulence of the glyceraldehyde-3-phosphate dehydrogenase homologues gapA and gapB in Staphylococcus aureus
Abstract
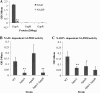
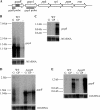
The Gram-positive bacterium Staphylococcus aureus contains two glyceraldehyde-3-phosphate dehydrogenase (GAPDH) homologues known as GapA and GapB. GapA has been characterized as a functional GAPDH protein, but currently there is no biological evidence for the role of GapB in metabolism in S. aureus. In this study we show through a number of complementary methods that S. aureus GapA is essential for glycolysis while GapB is essential in gluconeogenesis. These proteins are reciprocally regulated in response to glucose concentrations, and both are influenced by the glycolysis regulator protein GapR, which is the first demonstration of the role of this regulator in S. aureus and the first indication that GapR homologues control genes other than those within the glycolytic operon. Furthermore, we show that both GapA and GapB are important in the pathogenesis of S. aureus in a Galleria mellonella model of infection, showing for the first time in any bacteria that both glycolysis and gluconeogenesis have important roles in virulence.
Figures



References
-
- Boschi-Muller, S., S. Azza, D. Pollastro, C. Corbier, and G. Branlant. 1997. Comparative enzymatic properties of GapB-encoded erythrose-4-phosphate dehydrogenase of Escherichia coli and phosphorylating glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 272:15106-15112. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

