Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60
- PMID: 20876294
- PMCID: PMC2981324
- DOI: 10.1128/IAI.00516-10
Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60
Abstract
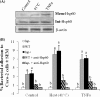
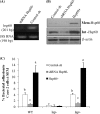
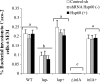
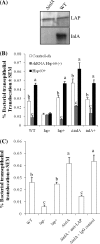
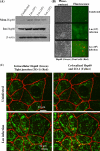
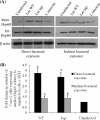
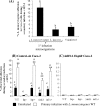
Listeria monocytogenes interaction with the intestinal epithelium is a key step in the infection process. We demonstrated that Listeria adhesion protein (LAP) promotes adhesion to intestinal epithelial cells and facilitates extraintestinal dissemination in vivo. The LAP receptor is a stress response protein, Hsp60, but the precise role for the LAP-Hsp60 interaction during Listeria infection is unknown. Here we investigated the influence of physiological stressors and Listeria infection on host Hsp60 expression and LAP-mediated bacterial adhesion, invasion, and transepithelial translocation in an enterocyte-like Caco-2 cell model. Stressors such as heat (41°C), tumor necrosis factor alpha (TNF-α) (100 U), and L. monocytogenes infection (10(4) to 10(6) CFU/ml) significantly (P < 0.05) increased plasma membrane and intracellular Hsp60 levels in Caco-2 cells and consequently enhanced LAP-mediated L. monocytogenes adhesion but not invasion of Caco-2 cells. In transepithelial translocation experiments, the wild type (WT) exhibited 2.7-fold more translocation through Caco-2 monolayers than a lap mutant, suggesting that LAP is involved in transepithelial translocation, potentially via a paracellular route. Short hairpin RNA (shRNA) suppression of Hsp60 in Caco-2 cells reduced WT adhesion and translocation 4.5- and 3-fold, respectively, while adhesion remained unchanged for the lap mutant. Conversely, overexpression of Hsp60 in Caco-2 cells enhanced WT adhesion and transepithelial translocation, but not those of the lap mutant. Furthermore, initial infection with a low dosage (10(6) CFU/ml) of L. monocytogenes increased plasma membrane and intracellular expression of Hsp60 significantly, which rendered Caco-2 cells more susceptible to subsequent LAP-mediated adhesion and translocation. These data provide insight into the role of LAP as a virulence factor during intestinal epithelial infection and pose new questions regarding the dynamics between the host stress response and pathogen infection.
Figures

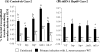

References
-
- Alvarez-Dominguez, C., J. A. Vazquez-Boland, E. Carrasco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect. Immun. 65:78-88. - PMC - PubMed
-
- Bocharov, A. V., T. G. Vishnyakova, I. N. Baranova, A. T. Remaley, A. P. Patterson, and T. L. Eggerman. 2000. Heat shock protein 60 is a high-affinity high-density lipoprotein binding protein. Biochem. Biophys. Res. Commun. 277:228-235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

