Ime1 and Ime2 are required for pseudohyphal growth of Saccharomyces cerevisiae on nonfermentable carbon sources
- PMID: 20876298
- PMCID: PMC2976428
- DOI: 10.1128/MCB.00390-10
Ime1 and Ime2 are required for pseudohyphal growth of Saccharomyces cerevisiae on nonfermentable carbon sources
Abstract
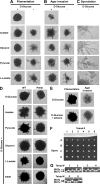
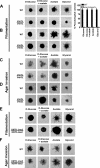
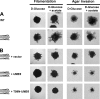
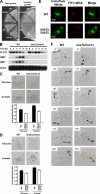
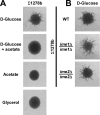
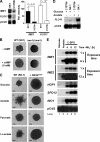
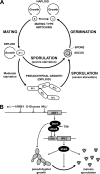
Pseudohyphal growth and meiosis are two differentiation responses to nitrogen starvation of diploid Saccharomyces cerevisiae. Nitrogen starvation in the presence of fermentable carbon sources is thought to induce pseudohyphal growth, whereas nitrogen and sugar starvation induces meiosis. In contrast to the genetic background routinely used to study pseudohyphal growth (Σ1278b), nonfermentable carbon sources stimulate pseudohyphal growth in the efficiently sporulating strain SK1. Pseudohyphal SK1 cells can exit pseudohyphal growth to complete meiosis. Two stimulators of meiosis, Ime1 and Ime2, are required for pseudohyphal growth of SK1 cells in the presence of nonfermentable carbon sources. Epistasis analysis suggests that Ime1 and Ime2 act in the same order in pseudohyphal growth as in meiosis. The different behaviors of strains SK1 and Σ1278b are in part attributable to differences in cyclic AMP (cAMP) signaling. In contrast to Σ1278b cells, hyperactivation of cAMP signaling using constitutively active Ras2(G19V) inhibited pseudohyphal growth in SK1 cells. Our data identify the SK1 genetic background as an alternative genetic background for the study of pseudohyphal growth and suggest an overlap between signaling pathways controlling pseudohyphal growth and meiosis. Based on these findings, we propose to include exit from pseudohyphal growth and entry into meiosis in the life cycle of S. cerevisiae.
Figures


Similar articles
-
The in vivo activity of Ime1, the key transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae, is inhibited by the cyclic AMP/protein kinase A signal pathway through the glycogen synthase kinase 3-beta homolog Rim11.Mol Cell Biol. 2004 Aug;24(16):6967-79. doi: 10.1128/MCB.24.16.6967-6979.2004. Mol Cell Biol. 2004. PMID: 15282298 Free PMC article.
-
Bipartite structure of an early meiotic upstream activation sequence from Saccharomyces cerevisiae.Mol Cell Biol. 1993 Apr;13(4):2172-81. doi: 10.1128/mcb.13.4.2172-2181.1993. Mol Cell Biol. 1993. PMID: 8455605 Free PMC article.
-
The S. cerevisiae nitrogen starvation-induced Yvh1p and Ptp2p phosphatases play a role in control of sporulation.Yeast. 1996 Sep 15;12(11):1135-51. doi: 10.1002/(sici)1097-0061(19960915)12:11<1135::aid-yea11>3.0.co;2-l. Yeast. 1996. PMID: 8896280
-
The Ime2 protein kinase family in fungi: more duties than just meiosis.Mol Microbiol. 2011 Apr;80(1):1-13. doi: 10.1111/j.1365-2958.2011.07575.x. Epub 2011 Mar 1. Mol Microbiol. 2011. PMID: 21306447 Review.
-
Transcriptional regulation of meiosis in budding yeast.Int Rev Cytol. 2003;224:111-71. doi: 10.1016/s0074-7696(05)24004-4. Int Rev Cytol. 2003. PMID: 12722950 Review.
Cited by
-
Rpl22 is required for IME1 mRNA translation and meiotic induction in S. cerevisiae.Cell Div. 2016 Jul 29;11:10. doi: 10.1186/s13008-016-0024-3. eCollection 2016. Cell Div. 2016. PMID: 27478489 Free PMC article.
-
Pleiotropic signaling pathways orchestrate yeast development.Curr Opin Microbiol. 2011 Dec;14(6):676-81. doi: 10.1016/j.mib.2011.09.004. Epub 2011 Sep 28. Curr Opin Microbiol. 2011. PMID: 21962291 Free PMC article. Review.
-
Protein Kinase Ime2 Is Required for Mycelial Growth, Conidiation, Osmoregulation, and Pathogenicity in Nematode-Trapping Fungus Arthrobotrys oligospora.Front Microbiol. 2020 Jan 14;10:3065. doi: 10.3389/fmicb.2019.03065. eCollection 2019. Front Microbiol. 2020. PMID: 31993040 Free PMC article.
-
The regulation of filamentous growth in yeast.Genetics. 2012 Jan;190(1):23-49. doi: 10.1534/genetics.111.127456. Genetics. 2012. PMID: 22219507 Free PMC article. Review.
-
The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2012 Mar;76(1):1-15. doi: 10.1128/MMBR.05010-11. Microbiol Mol Biol Rev. 2012. PMID: 22390969 Free PMC article. Review.
References
-
- Beullens, M., K. Mbonyi, L. Geerts, D. Gladines, K. Detremerie, A. W. Jans, and J. M. Thevelein. 1988. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 172:227-231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
