Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress
- PMID: 20876336
- PMCID: PMC3003804
- DOI: 10.1093/jxb/erq288
Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress
Abstract
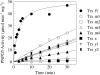
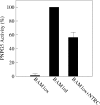
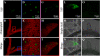
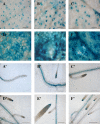
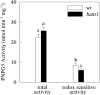
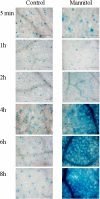
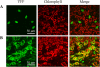
BAM1 is a plastid-targeted β-amylase of Arabidopsis thaliana specifically activated by reducing conditions. Among eight different chloroplast thioredoxin isoforms, thioredoxin f1 was the most efficient redox mediator, followed by thioredoxins m1, m2, y1, y2, and m4. Plastid-localized NADPH-thioredoxin reductase (NTRC) was also able partially to restore the activity of oxidized BAM1. Promoter activity of BAM1 was studied by reporter gene expression (GUS and YFP) in Arabidopsis transgenic plants. In young (non-flowering) plants, BAM1 was expressed both in leaves and roots, but expression in leaves was mainly restricted to guard cells. Compared with wild-type plants, bam1 knockout mutants were characterized by having more starch in illuminated guard cells and reduced stomata opening, suggesting that thioredoxin-regulated BAM1 plays a role in diurnal starch degradation which sustains stomata opening. Besides guard cells, BAM1 appears in mesophyll cells of young plants as a result of a strongly induced gene expression under osmotic stress, which is paralleled by an increase in total β-amylase activity together with its redox-sensitive fraction. Osmotic stress impairs the rate of diurnal starch accumulation in leaves of wild-type plants, but has no effect on starch accumulation in bam1 mutants. It is proposed that thioredoxin-regulated BAM1 activates a starch degradation pathway in illuminated mesophyll cells upon osmotic stress, similar to the diurnal pathway of starch degradation in guard cells that is also dependent on thioredoxin-regulated BAM1.
Figures
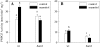
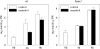
References
-
- Amodeo G, Talbott LD, Zeiger E. Use of potassium and sucrose by onion guard cells during a daily cycle of osmoregulation. Plant and Cell Physiology. 1996;37:575–579.
-
- Basu PS, Ali M, Chaturvedi SK. Osmotic adjustment increases water uptake, remobilization of assimilates and maintains photosynthesis in chickpea under drought. Indian Journal of Experimental Biology. 2007;45:261–267. - PubMed
-
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annual Review of Plant Biology. 2005;56:187–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

