Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis
- PMID: 20876338
- PMCID: PMC2971625
- DOI: 10.1104/pp.110.161869
Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis
Abstract
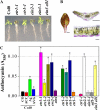
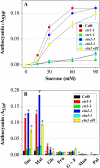
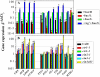
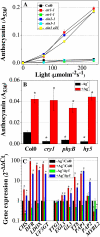
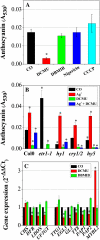
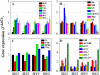
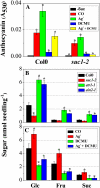
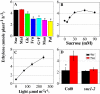
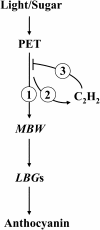
Anthocyanin accumulation is regulated negatively by ethylene signaling and positively by sugar and light signaling. However, the antagonistic interactions underlying these signalings remain to be elucidated fully. We show that ethylene inhibits anthocyanin accumulation induced by sucrose (Suc) and light by suppressing the expression of transcription factors that positively regulate anthocyanin biosynthesis, including GLABRA3, TRANSPARENT TESTA8, and PRODUCTION OF ANTHOCYANIN PIGMENT1, while stimulating the concomitant expression of the negative R3-MYB regulator MYBL2. Genetic analyses show that the ethylene-mediated suppression of anthocyanin accumulation is dependent upon ethylene signaling components responsible for the triple response. Furthermore, these positive and negative signaling pathways appear to be under photosynthetic control. Suc and light induction of anthocyanin accumulation was almost fully inhibited in wild-type Arabidopsis (Arabidopsis thaliana) ecotype Columbia and ethylene (ethylene response1 [etr1-1]) and light (long hypocotyl1 [hy1], cryptochrome1/2, and hy5) signaling mutants treated with the photosynthetic electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea. The transcript level of the sugar transporter gene SUC1 was enhanced in ecotype Columbia treated with the ethylene-binding inhibitor silver and in etr1-1, ethylene insensitive2 (ein2-1), and ein3 ein3-like1 mutants. In contrast, 3-(3,4-dichlorophenyl)-1,1-dimethylurea treatment reduced SUC1 expression, which indicates strongly that SUC1 represents an integrator for signals provided by sugar, light, and ethylene. SUC1 mutations lowered accumulations of anthocyanin pigment, soluble sugar content, and ethylene production in response to Suc and light signals. These data demonstrate that the suppression of SUC1 expression by ethylene inhibits Suc-induced anthocyanin accumulation in the presence of light and, hence, fine-tunes anthocyanin homeostasis.
Figures

Similar articles
-
Arabidopsis ROOT HAIR DEFECTIVE3 is involved in nitrogen starvation-induced anthocyanin accumulation.J Integr Plant Biol. 2015 Aug;57(8):708-21. doi: 10.1111/jipb.12320. Epub 2015 Feb 2. J Integr Plant Biol. 2015. PMID: 25494721
-
Calcium dependent sucrose uptake links sugar signaling to anthocyanin biosynthesis in Arabidopsis.Biochem Biophys Res Commun. 2013 Jan 11;430(2):634-9. doi: 10.1016/j.bbrc.2012.11.100. Epub 2012 Dec 4. Biochem Biophys Res Commun. 2013. PMID: 23220235
-
The ethylene signaling pathway has a negative impact on sucrose-induced anthocyanin accumulation in Arabidopsis.J Plant Res. 2011 Jan;124(1):193-200. doi: 10.1007/s10265-010-0354-1. Epub 2010 Jun 1. J Plant Res. 2011. PMID: 20514508
-
Mechanistic Insights in Ethylene Perception and Signal Transduction.Plant Physiol. 2015 Sep;169(1):85-95. doi: 10.1104/pp.15.00845. Epub 2015 Aug 5. Plant Physiol. 2015. PMID: 26246449 Free PMC article. Review.
-
Ethylene signaling in plants.J Biol Chem. 2020 May 29;295(22):7710-7725. doi: 10.1074/jbc.REV120.010854. Epub 2020 Apr 24. J Biol Chem. 2020. PMID: 32332098 Free PMC article. Review.
Cited by
-
Melatonin alters the secondary metabolite profile of grape berry skin by promoting VvMYB14-mediated ethylene biosynthesis.Hortic Res. 2021 Mar 1;8(1):43. doi: 10.1038/s41438-021-00478-2. Hortic Res. 2021. PMID: 33642583 Free PMC article.
-
Sugar signal mediates flavonoid biosynthesis in tea leaves.Hortic Res. 2022 Mar 14;9:uhac049. doi: 10.1093/hr/uhac049. eCollection 2022. Hortic Res. 2022. PMID: 35591928 Free PMC article.
-
Regulatory Functions of Cellular Energy Sensor SNF1-Related Kinase1 for Leaf Senescence Delay through ETHYLENE- INSENSITIVE3 Repression.Sci Rep. 2017 Jun 9;7(1):3193. doi: 10.1038/s41598-017-03506-1. Sci Rep. 2017. PMID: 28600557 Free PMC article.
-
MdSnRK1.1 interacts with MdJAZ18 to regulate sucrose-induced anthocyanin and proanthocyanidin accumulation in apple.J Exp Bot. 2017 May 17;68(11):2977-2990. doi: 10.1093/jxb/erx150. J Exp Bot. 2017. PMID: 28549152 Free PMC article.
-
Network analysis of the metabolome and transcriptome reveals novel regulation of potato pigmentation.J Exp Bot. 2016 Mar;67(5):1519-33. doi: 10.1093/jxb/erv549. Epub 2016 Jan 4. J Exp Bot. 2016. PMID: 26733692 Free PMC article.
References
-
- Ahmad M, Cashmore AR. (1997) The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J 11: 421–427 - PubMed
-
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R. (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40: 1072–1078 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

