Joint tissues amplify inflammation and alter their invasive behavior via leukotriene B4 in experimental inflammatory arthritis
- PMID: 20876351
- PMCID: PMC3690310
- DOI: 10.4049/jimmunol.1001258
Joint tissues amplify inflammation and alter their invasive behavior via leukotriene B4 in experimental inflammatory arthritis
Abstract
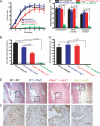
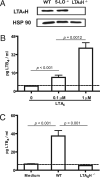
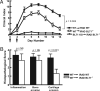
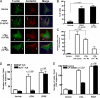
Mechanisms by which mesenchymal-derived tissue lineages participate in amplifying and perpetuating synovial inflammation in arthritis have been relatively underinvestigated and are therefore poorly understood. Elucidating these processes is likely to provide new insights into the pathogenesis of multiple diseases. Leukotriene B(4) (LTB(4)) is a potent proinflammatory lipid mediator that initiates and amplifies synovial inflammation in the K/BxN model of arthritis. We sought to elucidate mechanisms by which mesenchymal-derived fibroblast-like synoviocytes (FLSs) perpetuate synovial inflammation. We focused on the abilities of FLSs to contribute to LTB(4) synthesis and to respond to LTB(4) within the joint. Using a series of bone marrow chimeras generated from 5-lipoxygenase(-/-) and leukotriene A(4) (LTA(4)) hydrolase(-/-) mice, we demonstrate that FLSs generate sufficient levels of LTB(4) production through transcellular metabolism in K/BxN serum-induced arthritis to drive inflammatory arthritis. FLSs-which comprise the predominant lineage populating the synovial lining-are competent to metabolize exogenous LTA(4) into LTB(4) ex vivo. Stimulation of FLSs with TNF increased their capacity to generate LTB(4) 3-fold without inducing the expression of LTA(4) hydrolase protein. Moreover, LTB(4) (acting via LTB(4) receptor 1) was found to modulate the migratory and invasive activity of FLSs in vitro and also promote joint erosion by pannus tissue in vivo. Our results identify novel roles for FLSs and LTB(4) in joints, placing LTB(4) regulation of FLS biology at the center of a previously unrecognized amplification loop for synovial inflammation and tissue pathology.
Figures


Similar articles
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
HIF-2α-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models.Arthritis Res Ther. 2015 Oct 29;17:302. doi: 10.1186/s13075-015-0816-x. Arthritis Res Ther. 2015. PMID: 26510617 Free PMC article.
-
A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis.J Exp Med. 2006 Apr 17;203(4):829-35. doi: 10.1084/jem.20052349. Epub 2006 Mar 27. J Exp Med. 2006. PMID: 16567386 Free PMC article.
-
Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11.Immunol Rev. 2010 Jan;233(1):256-66. doi: 10.1111/j.0105-2896.2009.00854.x. Immunol Rev. 2010. PMID: 20193004 Review.
-
Role of leukotriene B4 receptors in rheumatoid arthritis.Autoimmun Rev. 2007 Nov;7(1):12-17. doi: 10.1016/j.autrev.2007.03.005. Epub 2007 Mar 30. Autoimmun Rev. 2007. PMID: 17967719 Free PMC article. Review.
Cited by
-
Persisting eicosanoid pathways in rheumatic diseases.Nat Rev Rheumatol. 2014 Apr;10(4):229-41. doi: 10.1038/nrrheum.2014.1. Epub 2014 Feb 11. Nat Rev Rheumatol. 2014. PMID: 24514915 Review.
-
The interrelationship between leukotriene B4 and leukotriene-A4-hydrolase in collagen/adjuvant-induced arthritis in rats.Biomed Res Int. 2014;2014:730421. doi: 10.1155/2014/730421. Epub 2014 Feb 20. Biomed Res Int. 2014. PMID: 24701582 Free PMC article.
-
Phenolic acid phenethylesters and their corresponding ketones: Inhibition of 5-lipoxygenase and stability in human blood and HepaRG cells.Pharmacol Res Perspect. 2019 Sep 13;7(5):e00524. doi: 10.1002/prp2.524. eCollection 2019 Oct. Pharmacol Res Perspect. 2019. PMID: 31523435 Free PMC article.
-
Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation.J Leukoc Biol. 2012 Feb;91(2):207-15. doi: 10.1189/jlb.0811402. Epub 2011 Nov 4. J Leukoc Biol. 2012. PMID: 22058421 Free PMC article. Review.
-
Identification of a novel autoantibody against self-vimentin specific in secondary Sjögren's syndrome.Arthritis Res Ther. 2018 Feb 12;20(1):30. doi: 10.1186/s13075-017-1508-5. Arthritis Res Ther. 2018. PMID: 29433534 Free PMC article.
References
-
- Henderson WR., Jr. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994;121:684–697. - PubMed
-
- Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007;405:379–395. - PubMed
-
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. - PubMed
-
- Werz O. 5-Lipoxygenase: cellular biology and molecular pharmacology. Curr. Drug Targets Inflamm. Allergy. 2002;1:23–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

