Heparan sulfate, including that in Bruch's membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration
- PMID: 20876352
- PMCID: PMC3639479
- DOI: 10.4049/jimmunol.0903596
Heparan sulfate, including that in Bruch's membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration
Abstract
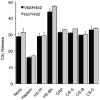
An imbalance between activation and inhibition of the complement system has been implicated in the etiologies of numerous common diseases. Allotypic variants of a key complement fluid-phase regulatory protein, complement factor H (CFH), are strongly associated with age-related macular degeneration (AMD), a leading cause of worldwide visual dysfunction, although its specific role in AMD pathogenesis is still not clear. CFH was isolated from individuals carrying combinations of two of the nonsynonymous coding variants most strongly associated with AMD risk, V62/H402 (risk haplotype variants), I62/Y402 (nonrisk haplotype variants), and V62/Y402. These proteins were used in two functional assays (cell surface- and fluid-phase-based) measuring cofactor activity of CFH in the factor I-mediated cleavage of C3b. Although no variant-specific differences in the cofactor activity were detected, when heparan sulfate (HS) was added to these assays, it accelerated the rate of C3b cleavage, and this effect could be modulated by degree of HS sulfation. Bruch's membrane/choroid, a site of tissue damage in AMD, contains high concentrations of glycosaminoglycans, including HS. Addition of human Bruch's membrane/choroid to the fluid-phase assay accelerated the C3b cleavage, and this effect was lost posttreatment of the tissue with heparinase III. Binding of CFH variants to Bruch's membrane/choroid isolated from elderly, non-AMD donor eyes, was similar, as was the functional activity of bound CFH. These findings refine our understanding of interactions of HS and complement and support the hypothesis that these interactions play a role in the transition between normal aging and AMD in Bruch's membrane/choroid.
Figures





References
-
- Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Molecular immunology. 2004;41:355–367. - PubMed
-
- DiScipio RG. Ultrastructures and interactions of complement factors H and I. J Immunol. 1992;149:2592–2599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

