A comprehensive analysis of deletions, multiplications, and copy number variations in PARK2
- PMID: 20876472
- PMCID: PMC3013490
- DOI: 10.1212/WNL.0b013e3181f4d832
A comprehensive analysis of deletions, multiplications, and copy number variations in PARK2
Abstract
Objectives: To perform a comprehensive population genetic study of PARK2. PARK2 mutations are associated with juvenile parkinsonism, Alzheimer disease, cancer, leprosy, and diabetes mellitus, yet ironically, there has been no comprehensive study of PARK2 in control subjects; and to resolve controversial association of PARK2 heterozygous mutations with Parkinson disease (PD) in a well-powered study.
Methods: We studied 1,686 control subjects (mean age 66.1 ± 13.1 years) and 2,091 patients with PD (mean onset age 58.3 ± 12.1 years). We tested for PARK2 deletions/multiplications/copy number variations (CNV) using semiquantitative PCR and multiplex ligation-dependent probe amplification, and validated the mutations by real-time quantitative PCR. Subjects were tested for point mutations previously. Association with PD was tested as PARK2 main effect, and in combination with known PD risk factors: SNCA, MAPT, APOE, smoking, and coffee intake.
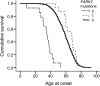
Results: A total of 0.95% of control subjects and 0.86% of patients carried a heterozygous CNV mutation. CNV mutations found in 16 control subjects were all in exons 1-4, sparing exons that encode functionally critical protein domains. Thirteen patients had 2 CNV mutations, 5 had 1 CNV and 1 point mutation, and 18 had 1 CNV mutation. Mutations found in patients spanned exons 2-9. In whites, having 1 CNV was not associated with increased risk (odds ratio 1.05, p = 0.89) or earlier onset of PD (64.7 ± 8.6 heterozygous vs 58.5 ± 11.8 normal).
Conclusions: This comprehensive population genetic study in control subjects fills the void for a PARK2 reference dataset. There is no compelling evidence for association of heterozygous PARK2 mutations, by themselves or in combination with known risk factors, with PD.
Figures
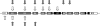

References
-
- Asakawa S, Tsunematsu K, Takayanagi A, et al. The genomic structure and promoter region of the human parkin gene. Biochem Biophys Res Commun 2001;286:863–868. - PubMed
-
- Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998;392:605–608. - PubMed
-
- Mira MT, Alcais A, Nguyen VT, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 2004;427:636–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5K01AG024329-02/AG/NIA NIH HHS/United States
- R01 NS065070/NS/NINDS NIH HHS/United States
- UL1-RR024140/RR/NCRR NIH HHS/United States
- R01 NS036960-09/NS/NINDS NIH HHS/United States
- RC2AG036528/AG/NIA NIH HHS/United States
- R01 AG 11762/AG/NIA NIH HHS/United States
- R01 MH089004/MH/NIMH NIH HHS/United States
- R01 AG033398-01/AG/NIA NIH HHS/United States
- U01AG032984/AG/NIA NIH HHS/United States
- P50 NS062684-01/NS/NINDS NIH HHS/United States
- R01 AG 21544/AG/NIA NIH HHS/United States
- NS R01-36960/NS/NINDS NIH HHS/United States
- R01 NS065070-01/NS/NINDS NIH HHS/United States
- 1 U01 NS50324-01A1/NS/NINDS NIH HHS/United States
- R01 NS 21062/NS/NINDS NIH HHS/United States
- AG 08017/AG/NIA NIH HHS/United States
- R01 NS057567-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
