Suppressor of cytokine signaling 3 inhibits LPS-induced IL-6 expression in osteoblasts by suppressing CCAAT/enhancer-binding protein {beta} activity
- PMID: 20876575
- PMCID: PMC2988329
- DOI: 10.1074/jbc.M110.132084
Suppressor of cytokine signaling 3 inhibits LPS-induced IL-6 expression in osteoblasts by suppressing CCAAT/enhancer-binding protein {beta} activity
Abstract
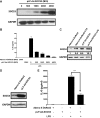
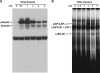
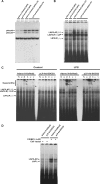
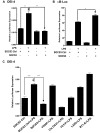
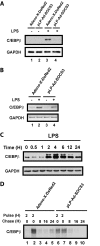
Suppressor of cytokine signaling 3 (SOCS3) is an important intracellular protein that inhibits cytokine signaling in numerous cell types and has been implicated in several inflammatory diseases. However, the expression and function of SOCS3 in osteoblasts are not known. In this study, we demonstrated that SOCS3 expression was transiently induced by LPS in osteoblasts, and apparently contributed to the inhibition of IL-6 induction by LPS treatment. We found that tyrosine 204 of the SOCS box, the SH2 domain, and the N-terminal kinase inhibitory region (KIR) of SOCS3 were all involved in its IL-6 inhibition. Furthermore, we demonstrated that CCAAT/enhancer-binding protein (C/EBP) β was activated by LPS (increased DNA binding activity), and played a key role in LPS-induced IL-6 expression in osteoblasts. We further provided the evidence that SOCS3 functioned as a negative regulator for LPS response in osteoblasts by suppressing C/EBPβ DNA binding activity. In addition, tyrosine 204 of the SOCS box, the SH2 domain, and the N-terminal kinase inhibitory region (KIR) of SOCS3 were all required for its C/EBPβ inhibition. These findings suggest that SOCS3 by interfering with C/EBPβ activation may have an important regulatory role during bone-associated inflammatory responses.
Figures





References
-
- Stoiber D., Kovarik P., Cohney S., Johnston J. A., Steinlein P., Decker T. (1999) J. Immunol. 163, 2640–2647 - PubMed
-
- Cassatella M. A., Gasperini S., Bovolenta C., Calzetti F., Vollebregt M., Scapini P., Marchi M., Suzuki R., Suzuki A., Yoshimura A. (1999) Blood 94, 2880–2889 - PubMed
-
- Bode J. G., Nimmesgern A., Schmitz J., Schaper F., Schmitt M., Frisch W., Häussinger D., Heinrich P. C., Graeve L. (1999) FEBS Lett. 463, 365–370 - PubMed
-
- Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien K. R., Yoshimura A. (2003) Nat. Immunol. 4, 551–556 - PubMed
-
- Kubo M., Hanada T., Yoshimura A. (2003) Nat. Immunol. 4, 1169–1176 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

