Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos
- PMID: 20876651
- PMCID: PMC2964097
- DOI: 10.1242/dev.051094
Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos
Abstract
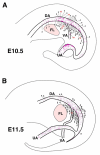
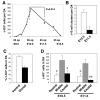
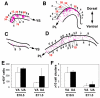
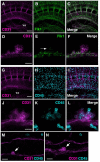
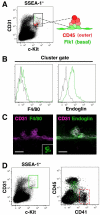
Hematopoietic cell clusters in the aorta of vertebrate embryos play a pivotal role in the formation of the adult blood system. Despite their importance, hematopoietic clusters have not been systematically quantitated or mapped because of technical limitations posed by the opaqueness of whole mouse embryos. Here, we combine an approach to make whole mouse embryos transparent, with multicolor marking, to allow observation of hematopoietic clusters using high-resolution 3-dimensional confocal microscopy. Our method provides the first complete map and temporal quantitation of all hematopoietic clusters in the mouse embryonic vasculature. We show that clusters peak in number at embryonic day 10.5, localize to specific vascular subregions and are heterogeneous, indicating a basal endothelial to non-basal (outer cluster) hematopoietic cell transition. Clusters enriched with the c-Kit(+)CD31(+)SSEA1(-) cell population contain functional hematopoietic progenitors and stem cells. Thus, three-dimensional cartography of transparent mouse embryos provides novel insight into the vascular subregions instrumental in hematopoietic progenitor/stem cell development, and represents an important technological advancement for comprehensive in situ hematopoietic cluster analysis.
Figures





Similar articles
-
Three-dimensional imaging of whole midgestation murine embryos shows an intravascular localization for all hematopoietic clusters.Blood. 2011 Jun 9;117(23):6132-4. doi: 10.1182/blood-2011-02-334037. Epub 2011 Apr 19. Blood. 2011. PMID: 21505195 Free PMC article.
-
Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse.Development. 2010 Dec;137(23):3941-52. doi: 10.1242/dev.051359. Epub 2010 Oct 27. Development. 2010. PMID: 20980401
-
Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs.Blood. 2013 Oct 3;122(14):2338-45. doi: 10.1182/blood-2012-12-470971. Epub 2013 Jul 17. Blood. 2013. PMID: 23863896 Free PMC article.
-
Genetic and functional characterization of isolated stromal cell lines from the aorta-gonado-mesonephros region.Ann N Y Acad Sci. 2005 Jun;1044:51-9. doi: 10.1196/annals.1349.007. Ann N Y Acad Sci. 2005. PMID: 15958697 Review.
-
Ontogenic emergence of definitive hematopoietic stem cells.Curr Opin Hematol. 2003 May;10(3):229-34. doi: 10.1097/00062752-200305000-00006. Curr Opin Hematol. 2003. PMID: 12690291 Review.
Cited by
-
Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo.Development. 2015 Mar 15;142(6):1050-61. doi: 10.1242/dev.119180. Development. 2015. PMID: 25758220 Free PMC article.
-
Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells.J Clin Invest. 2015 May;125(5):2032-45. doi: 10.1172/JCI80137. Epub 2015 Apr 13. J Clin Invest. 2015. PMID: 25866967 Free PMC article.
-
One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis.Cells. 2022 Mar 21;11(6):1061. doi: 10.3390/cells11061061. Cells. 2022. PMID: 35326511 Free PMC article. Review.
-
Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta.Blood. 2015 Jan 15;125(3):465-9. doi: 10.1182/blood-2014-07-588954. Epub 2014 Oct 9. Blood. 2015. PMID: 25301706 Free PMC article.
-
NR4A1 and NR4A2 orphan nuclear receptors regulate endothelial-to-hematopoietic transition in mouse hematopoietic stem cell specification.Development. 2024 Nov 15;151(22):dev201957. doi: 10.1242/dev.201957. Epub 2024 Nov 26. Development. 2024. PMID: 39589268 Free PMC article.
References
-
- Boisset J. C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116-120 - PubMed
-
- Cai Z., de Bruijn M., Ma X., Dortland B., Luteijn T., Downing R. J., Dzierzak E. (2000). Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 13, 423-431 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

