CMF70 is a subunit of the dynein regulatory complex
- PMID: 20876659
- PMCID: PMC2951471
- DOI: 10.1242/jcs.073817
CMF70 is a subunit of the dynein regulatory complex
Abstract
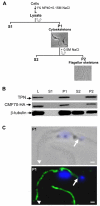
Flagellar motility drives propulsion of several important pathogens and is essential for human development and physiology. Motility of the eukaryotic flagellum requires coordinate regulation of thousands of dynein motors arrayed along the axoneme, but the proteins underlying dynein regulation are largely unknown. The dynein regulatory complex, DRC, is recognized as a focal point of axonemal dynein regulation, but only a single DRC subunit, trypanin/PF2, is currently known. The component of motile flagella 70 protein, CMF70, is broadly and uniquely conserved among organisms with motile flagella, suggesting a role in axonemal motility. Here we demonstrate that CMF70 is part of the DRC from Trypanosoma brucei. CMF70 is located along the flagellum, co-sediments with trypanin in sucrose gradients and co-immunoprecipitates with trypanin. RNAi knockdown of CMF70 causes motility defects in a wild-type background and suppresses flagellar paralysis in cells with central pair defects, thus meeting the functional definition of a DRC subunit. Trypanin and CMF70 are mutually conserved in at least five of six extant eukaryotic clades, indicating that the DRC was probably present in the last common eukaryotic ancestor. We have identified only the second known subunit of this ubiquitous dynein regulatory system, highlighting the utility of combined genomic and functional analyses for identifying novel subunits of axonemal sub-complexes.
Figures





Similar articles
-
Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system.Eukaryot Cell. 2006 Apr;5(4):696-711. doi: 10.1128/EC.5.4.696-711.2006. Eukaryot Cell. 2006. PMID: 16607017 Free PMC article.
-
Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement.J Cell Sci. 2007 May 1;120(Pt 9):1513-20. doi: 10.1242/jcs.004846. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405810
-
CMF22 is a broadly conserved axonemal protein and is required for propulsive motility in Trypanosoma brucei.Eukaryot Cell. 2013 Sep;12(9):1202-13. doi: 10.1128/EC.00068-13. Epub 2013 Jul 12. Eukaryot Cell. 2013. PMID: 23851336 Free PMC article.
-
Functional diversity of axonemal dyneins as assessed by in vitro and in vivo motility assays of Chlamydomonas mutants.Zoolog Sci. 2014 Oct;31(10):633-44. doi: 10.2108/zs140066. Zoolog Sci. 2014. PMID: 25284382 Review.
-
Force-Generating Mechanism of Axonemal Dynein in Solo and Ensemble.Int J Mol Sci. 2020 Apr 18;21(8):2843. doi: 10.3390/ijms21082843. Int J Mol Sci. 2020. PMID: 32325779 Free PMC article. Review.
Cited by
-
The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes.Mol Biol Cell. 2013 Apr;24(8):1134-52. doi: 10.1091/mbc.E12-11-0801. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427265 Free PMC article.
-
Nexin-Dynein regulatory complex component DRC7 but not FBXL13 is required for sperm flagellum formation and male fertility in mice.PLoS Genet. 2020 Jan 21;16(1):e1008585. doi: 10.1371/journal.pgen.1008585. eCollection 2020 Jan. PLoS Genet. 2020. PMID: 31961863 Free PMC article.
-
Centrin3 in trypanosomes maintains the stability of a flagellar inner-arm dynein for cell motility.Nat Commun. 2014 Jun 3;5:4060. doi: 10.1038/ncomms5060. Nat Commun. 2014. PMID: 24892844 Free PMC article.
-
Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme.Arch Biochem Biophys. 2011 Jun 15;510(2):93-100. doi: 10.1016/j.abb.2011.04.003. Epub 2011 Apr 14. Arch Biochem Biophys. 2011. PMID: 21513695 Free PMC article. Review.
-
Trypanosome doublet microtubule structures reveal flagellum assembly and motility mechanisms.Science. 2025 Mar 14;387(6739):eadr3314. doi: 10.1126/science.adr3314. Epub 2025 Mar 14. Science. 2025. PMID: 40080582
References
-
- Baron D. M., Kabututu Z. P., Hill K. L. (2007a). Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J. Cell Sci. 120, 1513-1520 - PubMed
-
- Baron D. M., Ralston K. S., Kabututu Z. P., Hill K. L. (2007b). Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J. Cell Sci. 120, 478-491 - PubMed
-
- Bastin P., Sherwin T., Gull K. (1998). Paraflagellar rod is vital for trypanosome motility. Nature 391, 548 - PubMed
-
- Bastin P., Pullen T. J., Sherwin T., Gull K. (1999). Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J. Cell Sci. 112, 3769-3777 - PubMed
-
- Bekker J. M., Colantonio J. R., Stephens A. D., Clarke W. T., King S. J., Hill K. L., Crosbie R. H. (2007). Direct interaction of Gas11 with microtubules: implications for the dynein regulatory complex. Cell Motil. Cytoskeleton 64, 461-473 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

