Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats
- PMID: 20876714
- PMCID: PMC3012184
- DOI: 10.2337/db10-0818
Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats
Abstract
Objective: Impairments in mitochondrial function have been proposed to play a role in the etiology of diabetic sensory neuropathy. We tested the hypothesis that mitochondrial dysfunction in axons of sensory neurons in type 1 diabetes is due to abnormal activity of the respiratory chain and an altered mitochondrial proteome.
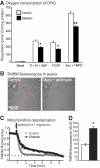
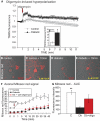
Research design and methods: Proteomic analysis using stable isotope labeling with amino acids in cell culture (SILAC) determined expression of proteins in mitochondria from dorsal root ganglia (DRG) of control, 22-week-old streptozotocin (STZ)-diabetic rats, and diabetic rats treated with insulin. Rates of oxygen consumption and complex activities in mitochondria from DRG were measured. Fluorescence imaging of axons of cultured sensory neurons determined the effect of diabetes on mitochondrial polarization status, oxidative stress, and mitochondrial matrix-specific reactive oxygen species (ROS).
Results: Proteins associated with mitochondrial dysfunction, oxidative phosphorylation, ubiquinone biosynthesis, and the citric acid cycle were downregulated in diabetic samples. For example, cytochrome c oxidase subunit IV (COX IV; a complex IV protein) and NADH dehydrogenase Fe-S protein 3 (NDUFS3; a complex I protein) were reduced by 29 and 36% (P < 0.05), respectively, in diabetes and confirmed previous Western blot studies. Respiration and mitochondrial complex activity was significantly decreased by 15 to 32% compared with control. The axons of diabetic neurons exhibited oxidative stress and depolarized mitochondria, an aberrant adaption to oligomycin-induced mitochondrial membrane hyperpolarization, but reduced levels of intramitochondrial superoxide compared with control.
Conclusions: Abnormal mitochondrial function correlated with a downregulation of mitochondrial proteins, with components of the respiratory chain targeted in lumbar DRG in diabetes. The reduced activity of the respiratory chain was associated with diminished superoxide generation within the mitochondrial matrix and did not contribute to oxidative stress in axons of diabetic neurons. Alternative pathways involving polyol pathway activity appear to contribute to raised ROS in axons of diabetic neurons under high glucose concentration.
Figures


References
-
- Vincent AM, Russell JW, Low P, Feldman EL: Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004;25:612–628 - PubMed
-
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 - PubMed
-
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M: Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 2000;97:12222–12226 - PMC - PubMed
-
- Coppey LJ, Gellett JS, Davidson EP, Yorek MA: Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res 2003;37:33–40 - PubMed
-
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF: Analysis of glucose metabolism in diabetic rat retinas. Am J Physiol Endocrinol Metab 2006;290:E1057–1067 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

