Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition
- PMID: 20876730
- PMCID: PMC2955813
- DOI: 10.1158/1940-6207.CAPR-10-0030
Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition
Abstract
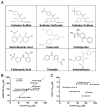
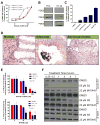
Nonsteroidal anti-inflammatory drugs (NSAID) display promising antineoplastic activity, but toxicity resulting from cyclooxygenase (COX) inhibition limits their clinical use for chemoprevention. Studies suggest that the mechanism may be COX independent, although alternative targets have not been well defined. Here, we show that the NSAID sulindac sulfide (SS) inhibits cyclic guanosine 3',5'-monophosphate (cGMP) phosphodiesterase (PDE) activity in colon tumor cell lysates at concentrations that inhibit colon tumor cell growth in vitro and in vivo. A series of chemically diverse NSAIDs also inhibited cGMP hydrolysis at concentrations that correlate with their potency to inhibit colon tumor cell growth, whereas no correlation was observed with COX-2 inhibition. Consistent with its selectivity for inhibiting cGMP hydrolysis compared with cyclic AMP hydrolysis, SS inhibited the cGMP-specific PDE5 isozyme and increased cGMP levels in colon tumor cells. Of numerous PDE isozyme-specific inhibitors evaluated, only the PDE5-selective inhibitor MY5445 inhibited colon tumor cell growth. The effects of SS and MY5445 on cell growth were associated with inhibition of β-catenin-mediated transcriptional activity to suppress the synthesis of cyclin D and survivin, which regulate tumor cell proliferation and apoptosis, respectively. SS had minimal effects on cGMP PDE activity in normal colonocytes, which displayed reduced sensitivity to SS and did not express PDE5. PDE5 was found to be overexpressed in colon tumor cell lines as well as in colon adenomas and adenocarcinomas compared with normal colonic mucosa. These results suggest that PDE5 inhibition, cGMP elevation, and inhibition of β-catenin transcriptional activity may contribute to the chemopreventive properties of certain NSAIDs.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



References
-
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002 Feb 20;94(4):252–66. - PubMed
-
- Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002 Mar;3(3):166–74. - PubMed
-
- Alberts DS, Hixson L, Ahnen D, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biochem Suppl. 1995;22:18–23. - PubMed
-
- Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997 Oct;3(10):1679–83. - PubMed
-
- Hanif R, Pittas A, Feng Y, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996 Jul 26;52(2):237–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

