Rapamycin regulates stearoyl CoA desaturase 1 expression in breast cancer
- PMID: 20876744
- PMCID: PMC2965451
- DOI: 10.1158/1535-7163.MCT-09-0980
Rapamycin regulates stearoyl CoA desaturase 1 expression in breast cancer
Abstract
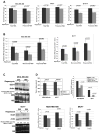
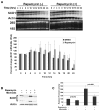
Mammalian target of rapamycin (mTOR) signaling is a central regulator of protein translation, cell growth, and metabolism. Alterations of the mTOR signaling pathway are common in cancer, making mTOR a promising therapeutic target. In clinical trials, rapamycin analogs have shown modest response rates for most cancer types, including breast cancer. Therefore, there is an urgent need to better understand the mechanism of action of rapamycin to improve patient selection and to monitor pathway inhibition. To identify novel pharmacodynamic markers of rapamycin activity, we carried out transcriptional profiling of total and polysome-associated RNA in three breast cancer cell lines representing different subtypes. In all three cell lines, we found that rapamycin significantly decreased polysome-associated mRNA for stearoyl-CoA desaturase 1 (SCD1), the rate-limiting enzyme in monounsaturated fatty acid synthesis. Activators of mTOR increased SCD1 protein expression, whereas rapamycin, LY294002, and BEZ235 decreased SCD1 protein expression. Rapamycin decreased total SCD1 RNA expression without inducing a significant decline in its relative polysomal recruitment (polysome/total ratio). Rapamycin did not alter SCD1 mRNA stability. Instead, rapamycin inhibited SCD1 promoter activity and decreased expression of mature transcription factor sterol regulatory element binding protein 1 (SREBP1). Eukaryotic initiation factor 4E (eIF4E) small interfering RNA (siRNA) decreased both SCD1 and SREBP1 expression, suggesting that SCD1 may be regulated through the mTOR/eIF4E-binding protein 1 axis. Furthermore, SCD1 siRNA knockdown inhibited breast cancer cell growth, whereas overexpression increased growth. Taken together these findings show that rapamycin decreases SCD1 expression, establishing an important link between cell signaling and cancer cell fatty acid synthesis and growth.
Conflict of interest statement
Figures




References
-
- Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6. - PubMed
-
- Noh WC, Mondesire WH, Peng J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–23. - PubMed
-
- Mondesire WH, Jian W, Zhang H, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–42. - PubMed
-
- Yu K, Toral-Barza L, Discafani C, et al. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–58. - PubMed
-
- Meric-Bernstam F, Esteva FJ. Potential role of mammalian target of rapamycin inhibitors in breast cancer therapy. Clin Breast Cancer. 2005;6:357–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

